BenevolentAI Investor Day Presentation Deck
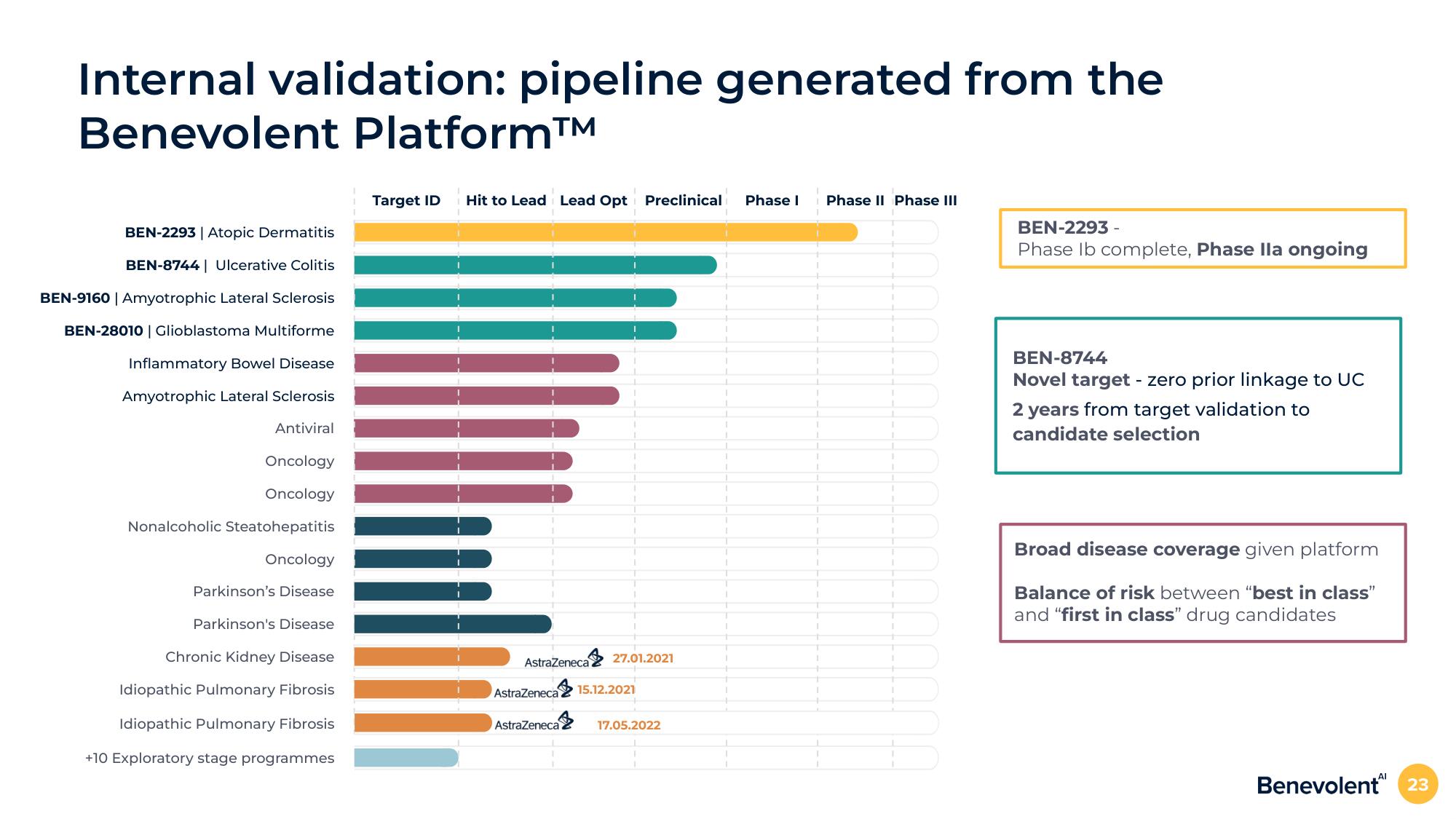
Internal validation: pipeline generated from the
Benevolent Platform™
BEN-2293 | Atopic Dermatitis
BEN-8744 | Ulcerative Colitis
BEN-9160 | Amyotrophic Lateral Sclerosis
BEN-28010 | Glioblastoma Multiforme
Inflammatory Bowel Disease
Amyotrophic Lateral Sclerosis
Antiviral
Oncology
Oncology
Nonalcoholic Steatohepatitis
Oncology
Parkinson's Disease
Parkinson's Disease
Chronic Kidney Disease
Idiopathic Pulmonary Fibrosis
Idiopathic Pulmonary Fibrosis
+10 Exploratory stage programmes
Target ID
Hit to Lead Lead Opt Preclinical Phase I
AstraZeneca
27.01.2021
AstraZeneca 15.12.2021
AstraZeneca 17.05.2022
Phase II Phase III
BEN-2293 -
Phase lb complete, Phase Ila ongoing
BEN-8744
Novel target - zero prior linkage to UC
2 years from target validation to
candidate selection
Broad disease coverage given platform
Balance of risk between "best in class"
and "first in class" drug candidates
Benevolent 23View entire presentation