Investor Presentation
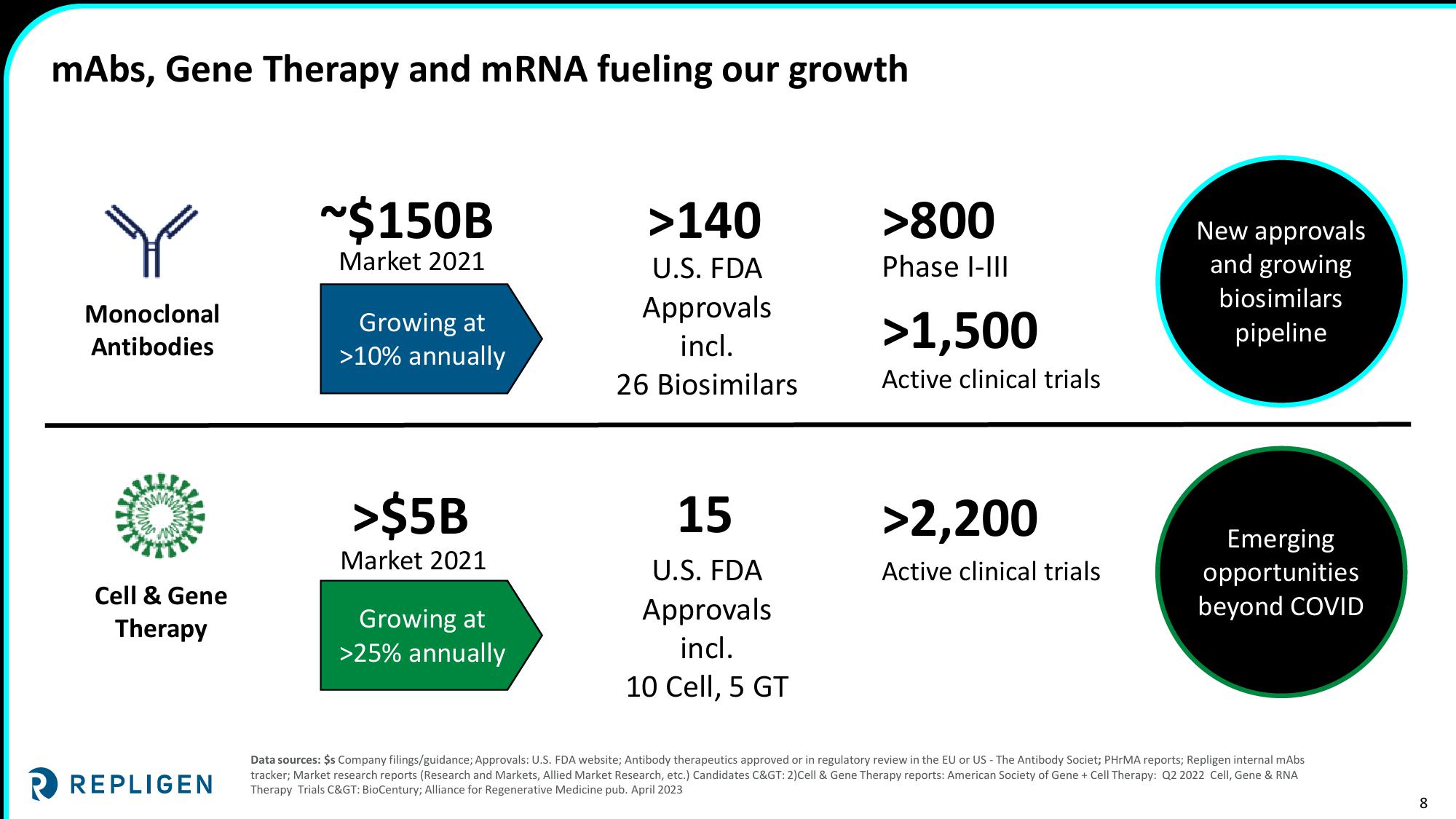
mAbs, Gene Therapy and mRNA fueling our growth
Monoclonal
Antibodies
Cell & Gene
Therapy
R REPLIGEN
~$150B
Market 2021
Growing at
>10% annually
>$5B
Market 2021
Growing at
>25% annually
>140
U.S. FDA
Approvals
incl.
26 Biosimilars
15
U.S. FDA
Approvals
incl.
10 Cell, 5 GT
>800
Phase I-III
>1,500
Active clinical trials
>2,200
Active clinical trials
New approvals
and growing
biosimilars
pipeline
Emerging
opportunities
beyond COVID
Data sources: $s Company filings/guidance; Approvals: U.S. FDA website; Antibody therapeutics approved or in regulatory review in the EU or US - The Antibody Societ; PHrMA reports; Repligen internal mAbs
tracker; Market research reports (Research and Markets, Allied Market Research, etc.) Candidates C>: 2) Cell & Gene Therapy reports: American Society of Gene + Cell Therapy: Q2 2022 Cell, Gene & RNA
Therapy Trials C>: BioCentury; Alliance for Regenerative Medicine pub. April 2023
8View entire presentation