Ocuphire Pharma Results Presentation Deck
9
CO
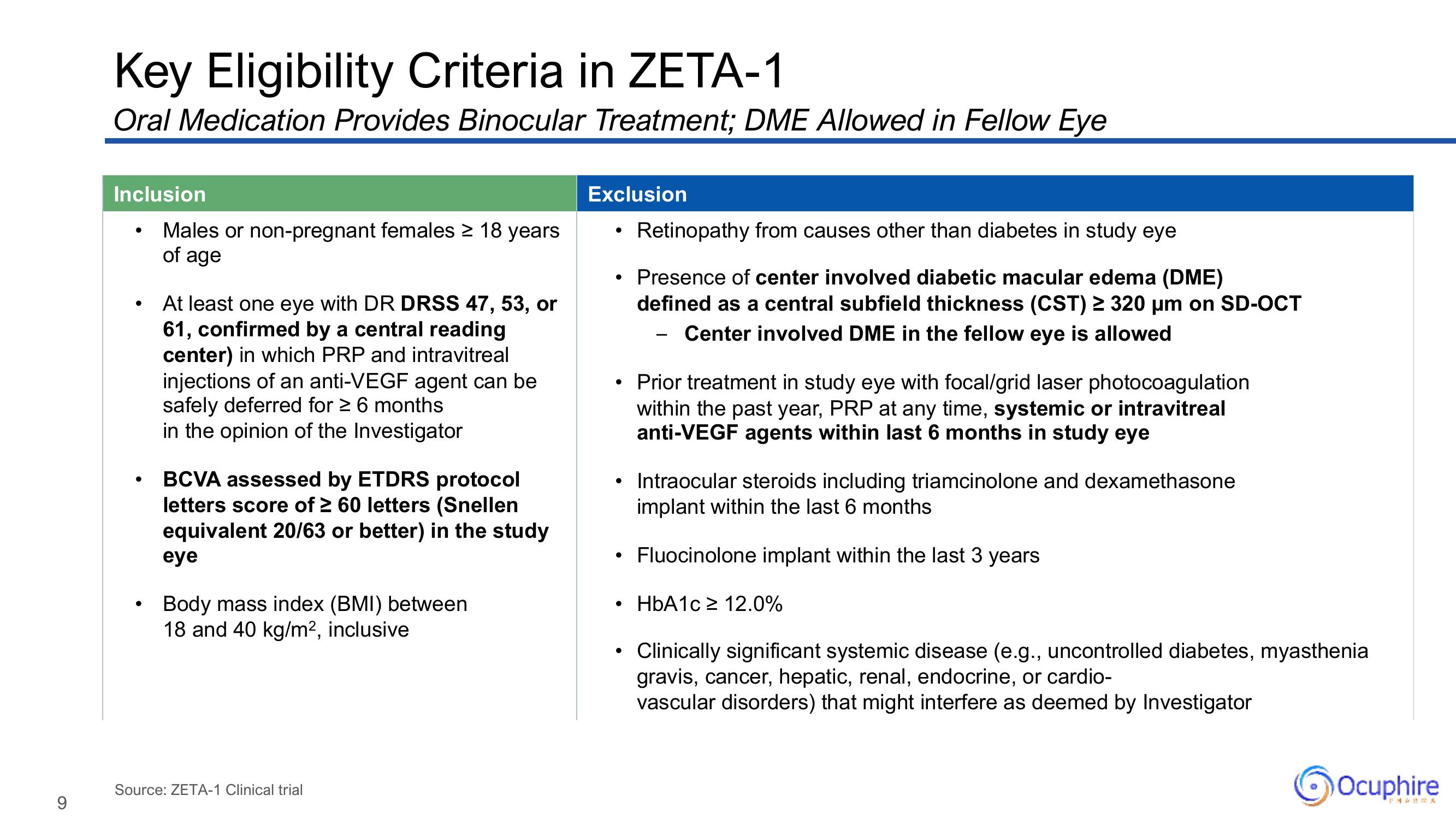
Key Eligibility Criteria in ZETA-1
Oral Medication Provides Binocular Treatment; DME Allowed in Fellow Eye
Inclusion
●
●
●
Males or non-pregnant females ≥ 18 years
of age
At least one eye with DR DRSS 47, 53, or
61, confirmed by a central reading
center) in which PRP and intravitreal
injections of an anti-VEGF agent can be
safely deferred for ≥ 6 months
in the opinion of the Investigator
BCVA assessed by ETDRS protocol
letters score of ≥ 60 letters (Snellen
equivalent 20/63 or better) in the study
eye
Body mass index (BMI) between
18 and 40 kg/m², inclusive
Source: ZETA-1 Clinical trial
Exclusion
●
• Prior treatment in study eye with focal/grid laser photocoagulation
within the past year, PRP at any time, systemic or intravitreal
anti-VEGF agents within last 6 months in study eye
●
●
Retinopathy from causes other than diabetes in study eye
Presence of center involved diabetic macular edema (DME)
defined as a central subfield thickness (CST) ≥ 320 µm on SD-OCT
Center involved DME in the fellow eye is allowed
●
Intraocular steroids including triamcinolone and dexamethasone
implant within the last 6 months
Fluocinolone implant within the last 3 years
HbA1c ≥ 12.0%
Clinically significant systemic disease (e.g., uncontrolled diabetes, myasthenia
gravis, cancer, hepatic, renal, endocrine, or cardio-
vascular disorders) that might interfere as deemed by Investigator
OcuphireView entire presentation