BioAtla IPO Presentation Deck
BA3021: Encouraging Results in Stage IV PD-1 Melanoma Patient
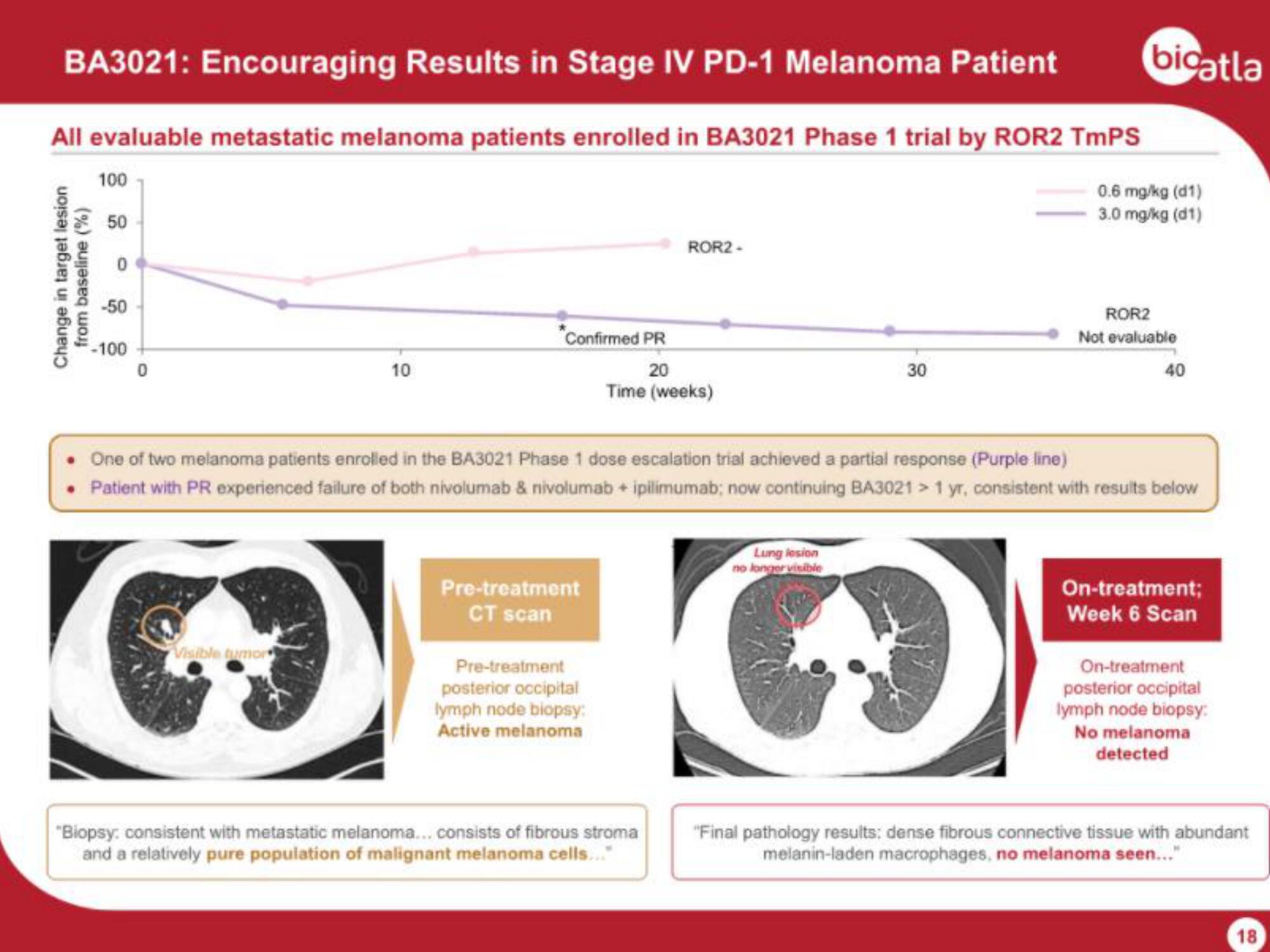
All evaluable metastatic melanoma patients enrolled in BA3021 Phase 1 trial by ROR2 TmPS
0.6 mg/kg (d1)
3.0 mg/kg (d1)
Change in target lesion
from baseline (%)
100
50
0
-50
-100
0
10
O
Visible tumor
Confirmed PR
20
Time (weeks)
Pre-treatment
CT scan
ROR2-
Pre-treatment
posterior occipital
lymph node biopsy:
Active melanoma
"Biopsy: consistent with metastatic melanoma... consists of fibrous stroma
and a relatively pure population of malignant melanoma cells...
One of two melanoma patients enrolled in the BA3021 Phase 1 dose escalation trial achieved a partial response (Purple line)
• Patient with PR experienced failure of both nivolumab & nivolumab + ipilimumab; now continuing BA3021 > 1 yr, consistent with results below
bicatla
30
Lung lesion
no longer visible
ROR2
Not evaluable
40
On-treatment;
Week 6 Scan
On-treatment
posterior occipital
lymph node biopsy:
No melanoma
detected
"Final pathology results: dense fibrous connective tissue with abundant
melanin-laden macrophages, no melanoma seen..."
18View entire presentation