BioNTech Investor Day Presentation Deck
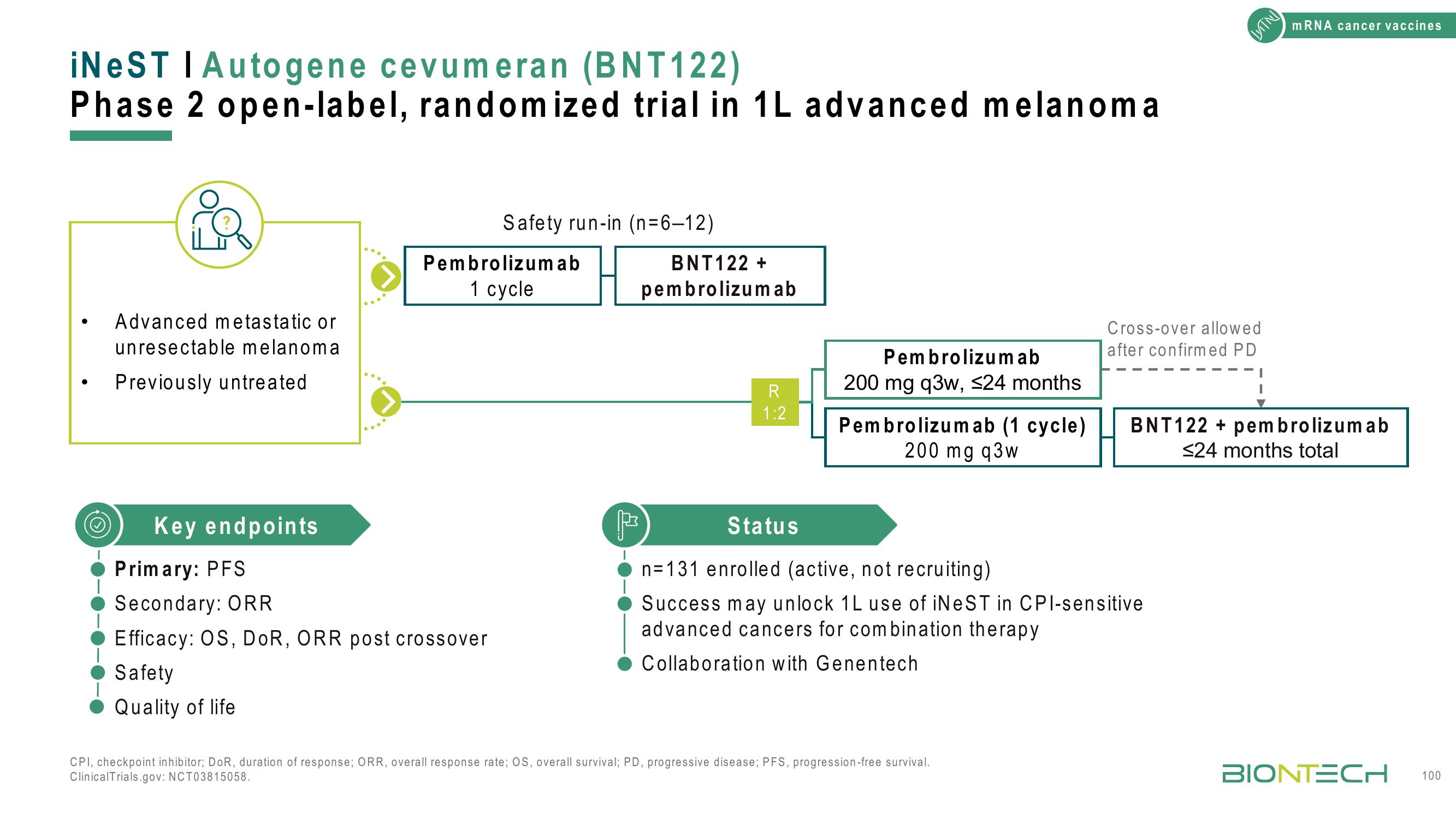
iNeST I Autogene cevumeran (BNT122)
Phase 2 open-label, randomized trial in 1L advanced melanoma
Ⓒ
Advanced metastatic or
unresectable melanoma
Previously untreated
Key endpoints
Safety run-in (n=6-12)
Pembrolizumab
1 cycle
Primary: PFS
Secondary: ORR
Efficacy: OS, DOR, ORR post crossover
Safety
Quality of life
BNT122 +
pembrolizumab
R
1:2
Pembrolizumab
200 mg q3w, ≤24 months
Pembrolizumab (1 cycle)
200 mg q3w
Cross-over allowed
after confirmed PD
CPI, checkpoint inhibitor; DoR, duration of response; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
Clinical Trials.gov: NCT03815058.
Status
n=131 enrolled (active, not recruiting)
Success may unlock 1L use of iNeST in CPI-sensitive
advanced cancers for combination therapy
Collaboration with Genentech
NUM
mRNA cancer vaccines
BNT122 + pembrolizumab
≤24 months total
BIONTECH
100View entire presentation