Neumora Therapeutics IPO Presentation Deck
Navacaprant: Well Tolerated with Lower TEAE Incidence Rate Compared to Placebo
●
●
●
Navacaprant was well tolerated and demonstrated lower
TEAE incidence rate compared to placebo:
- Overall discontinuation rates were higher on placebo compared
to navacaprant (29% navacaprant and 37% placebo)
- Incidence rates of TEAEs were 35% (navacaprant) and
44% (placebo)
Discontinuation rates related to TEAEs were higher on placebo
compared to navacaprant (1% navacaprant and
12% placebo)
The most common (>2%) TEAES in the navacaprant group
were mild to moderate in severity
Navacaprant was not associated with common side effects of
current antidepressant therapies - such
as weight gain or sexual dysfunction
No evidence of suicidal behavior was identified as assessed
the Columbia Suicide Severity Rating Scale
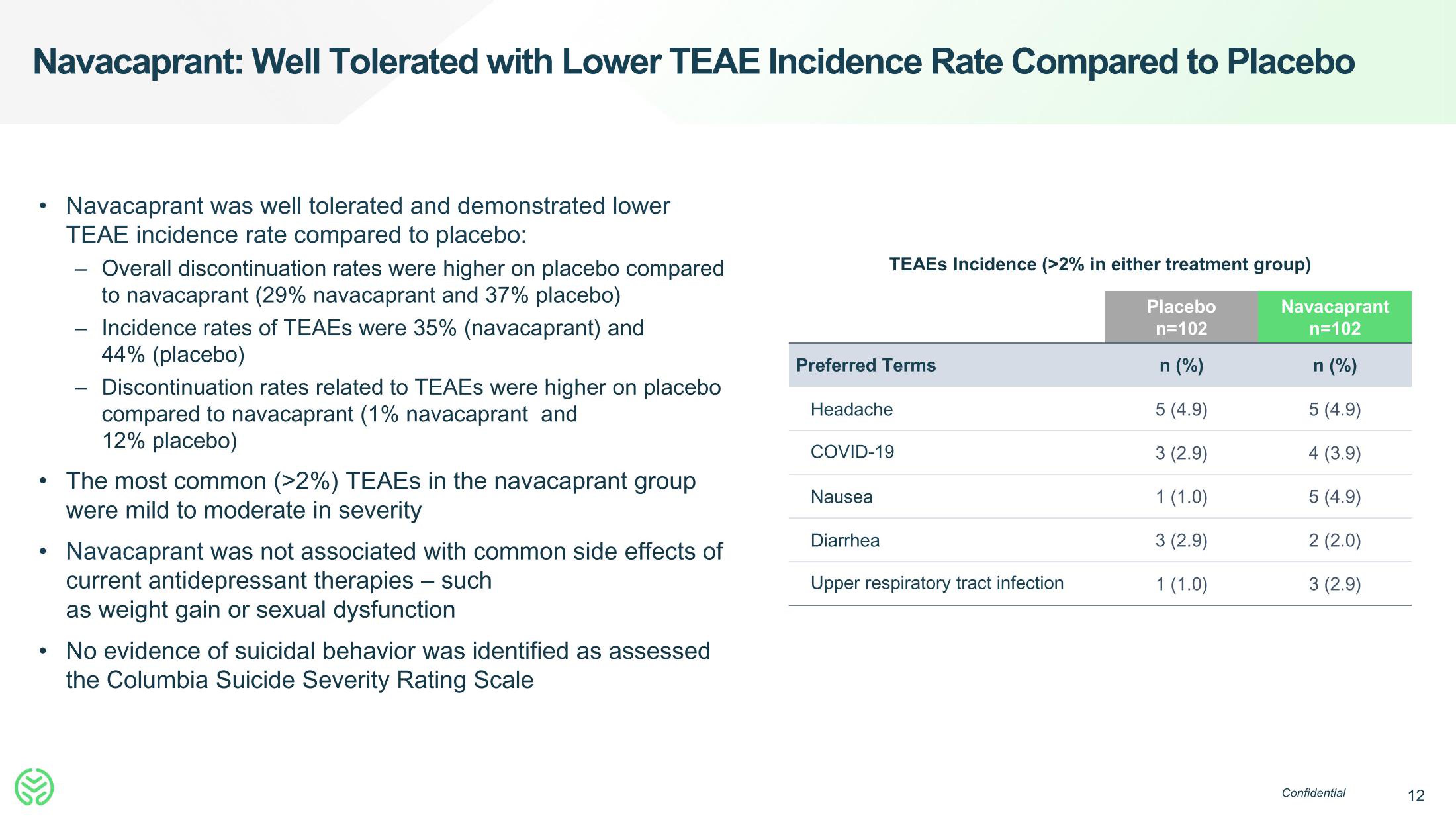
Preferred Terms
TEAES Incidence (>2% in either treatment group)
Placebo
n=102
n (%)
5 (4.9)
3 (2.9)
1 (1.0)
3 (2.9)
1 (1.0)
Headache
COVID-19
Nausea
Diarrhea
Upper respiratory tract infection
Navacaprant
n=102
n (%)
5 (4.9)
4 (3.9)
5 (4.9)
2 (2.0)
3 (2.9)
Confidential
12View entire presentation