BioNTech Results Presentation Deck
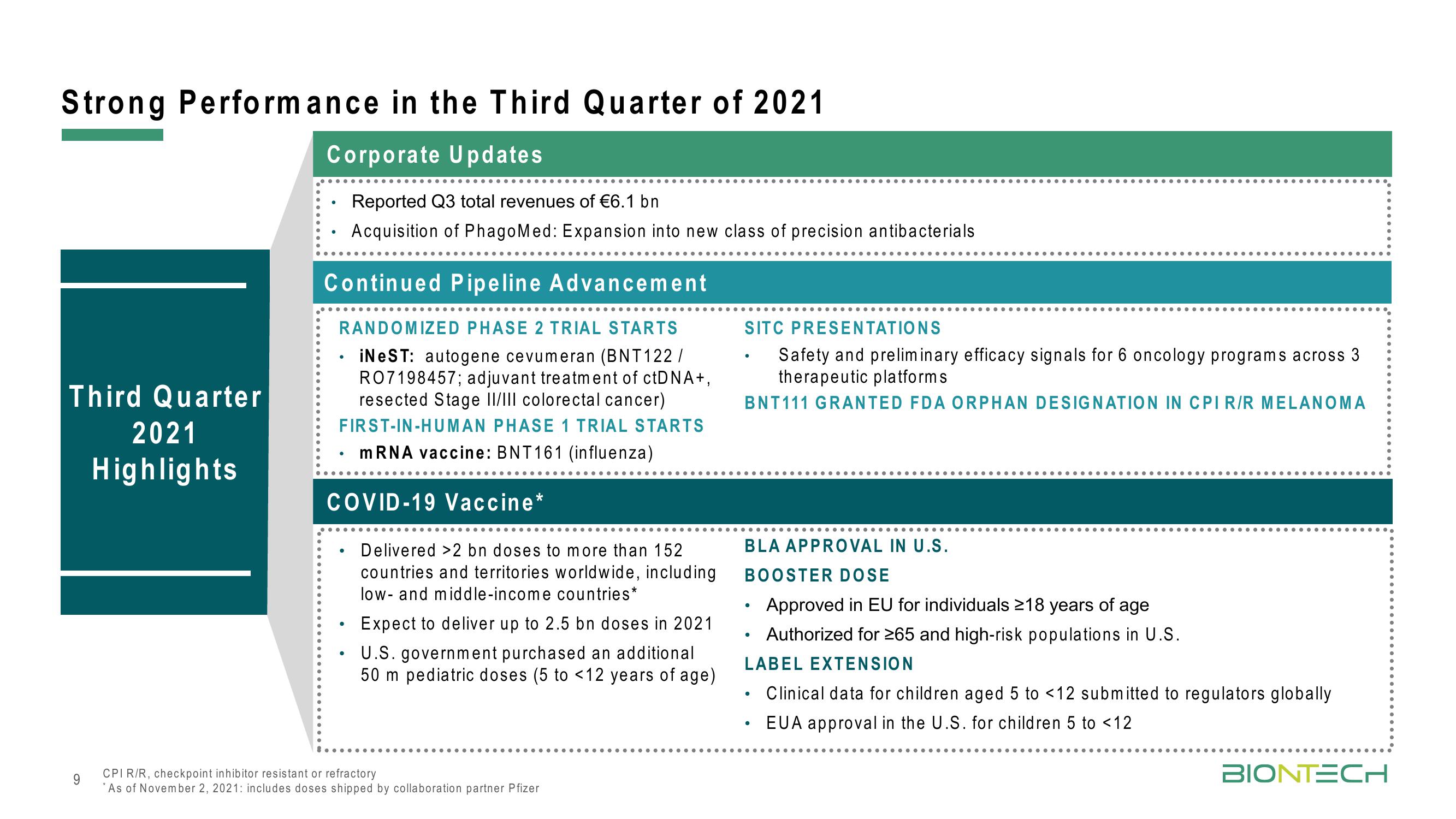
Strong Performance in the Third Quarter of 2021
Corporate Updates
Reported Q3 total revenues of €6.1 bn
Acquisition of PhagoMed: Expansion into new class of precision antibacterials
Third Quarter
2021
Highlights
9
Continued Pipeline Advancement
RANDOMIZED PHASE 2 TRIAL STARTS
iNeST: autogene cevumeran (BNT 122/
RO7198457; adjuvant treatment of ctDNA+,
resected Stage II/III colorectal cancer)
FIRST-IN-HUMAN PHASE 1 TRIAL STARTS
mRNA vaccine: BNT 161 (influenza)
COVID-19 Vaccine*
Delivered >2 bn doses to more than 152
countries and territories worldwide, including
low- and middle-income countries*
●
●
●
Expect to deliver up to 2.5 bn doses in 2021
U.S. government purchased an additional
50 m pediatric doses (5 to <12 years of age)
CPI R/R, checkpoint inhibitor resistant or refractory
"As of November 2, 2021: includes doses shipped by collaboration partner Pfizer
SITC PRESENTATIONS
Safety and preliminary efficacy signals for 6 oncology programs across 3
therapeutic platforms.
BNT111 GRANTED FDA ORPHAN DESIGNATION IN CPI R/R MELANOMA
BLA APPROVAL IN U.S.
BOOSTER DOSE
Approved in EU for individuals ≥18 years of age
Authorized for ≥65 and high-risk populations in U.S.
LABEL EXTENSION
●
●
●
Clinical data for children aged 5 to <12 submitted to regulators globally
EUA approval in the U.S. for children 5 to <12
BIONTECHView entire presentation