BenevolentAI Investor Presentation Deck
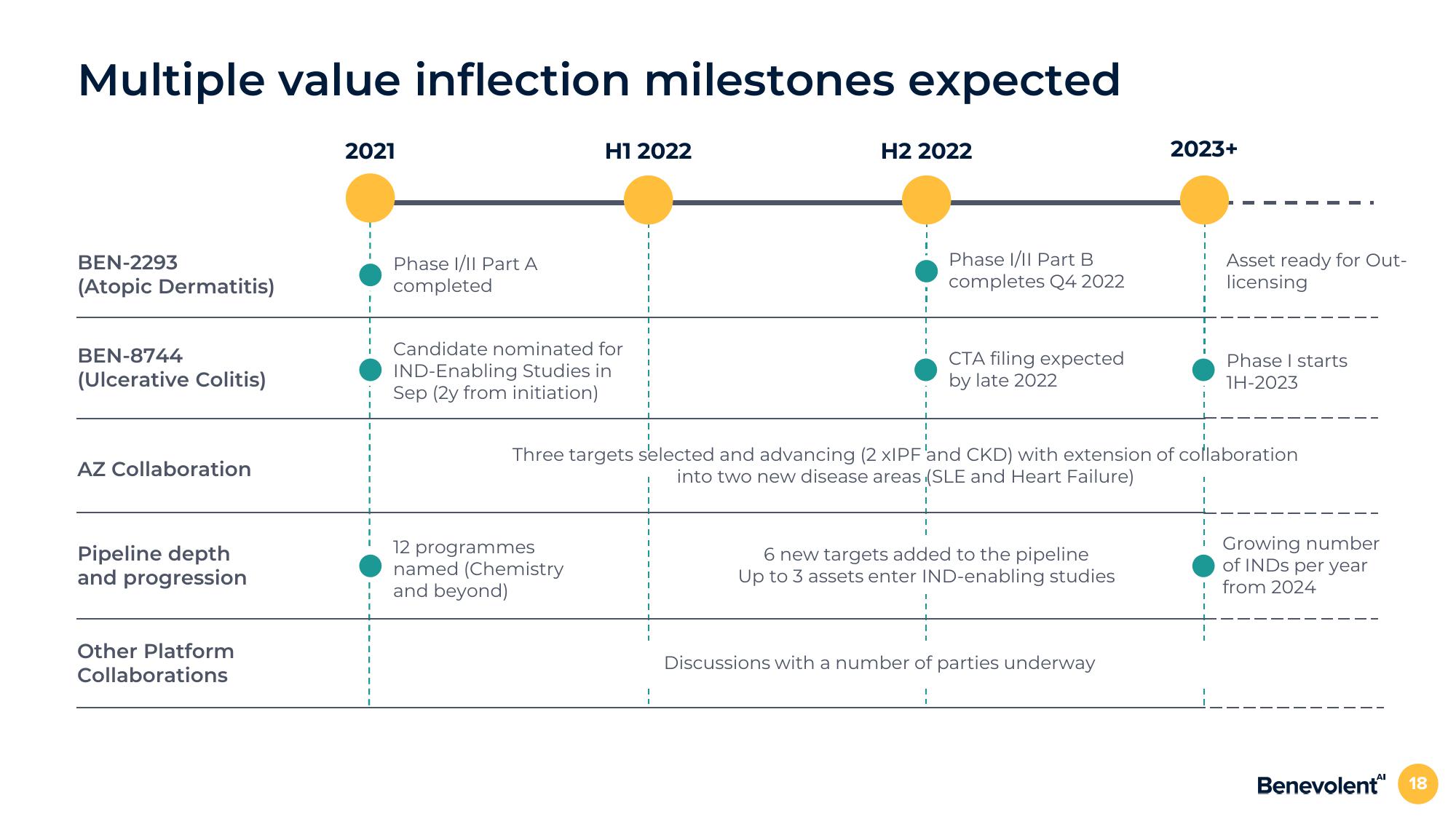
Multiple value inflection milestones expected
BEN-2293
(Atopic Dermatitis)
BEN-8744
(Ulcerative Colitis)
AZ Collaboration
Pipeline depth
and progression
Other Platform
Collaborations
2021
I
Phase I/II Part A
completed
H1 2022
Candidate nominated for
IND-Enabling Studies in
Sep (2y from initiation)
12 programmes
named (Chemistry
and beyond)
H2 2022
Phase I/II Part B
completes Q4 2022
CTA filing expected
by late 2022
6 new targets added to the pipeline
Up to 3 assets enter IND-enabling studies
2023+
Three targets selected and advancing (2 xIPF 'and CKD) with extension of collaboration
into two new disease areas (SLE and Heart Failure)
Discussions with a number of parties underway
Asset ready for Out-
licensing
I
I
Phase I starts
1H-2023
Growing number
of INDS per year
from 2024
Benevolent 18View entire presentation