Kymera Investor Day Presentation Deck
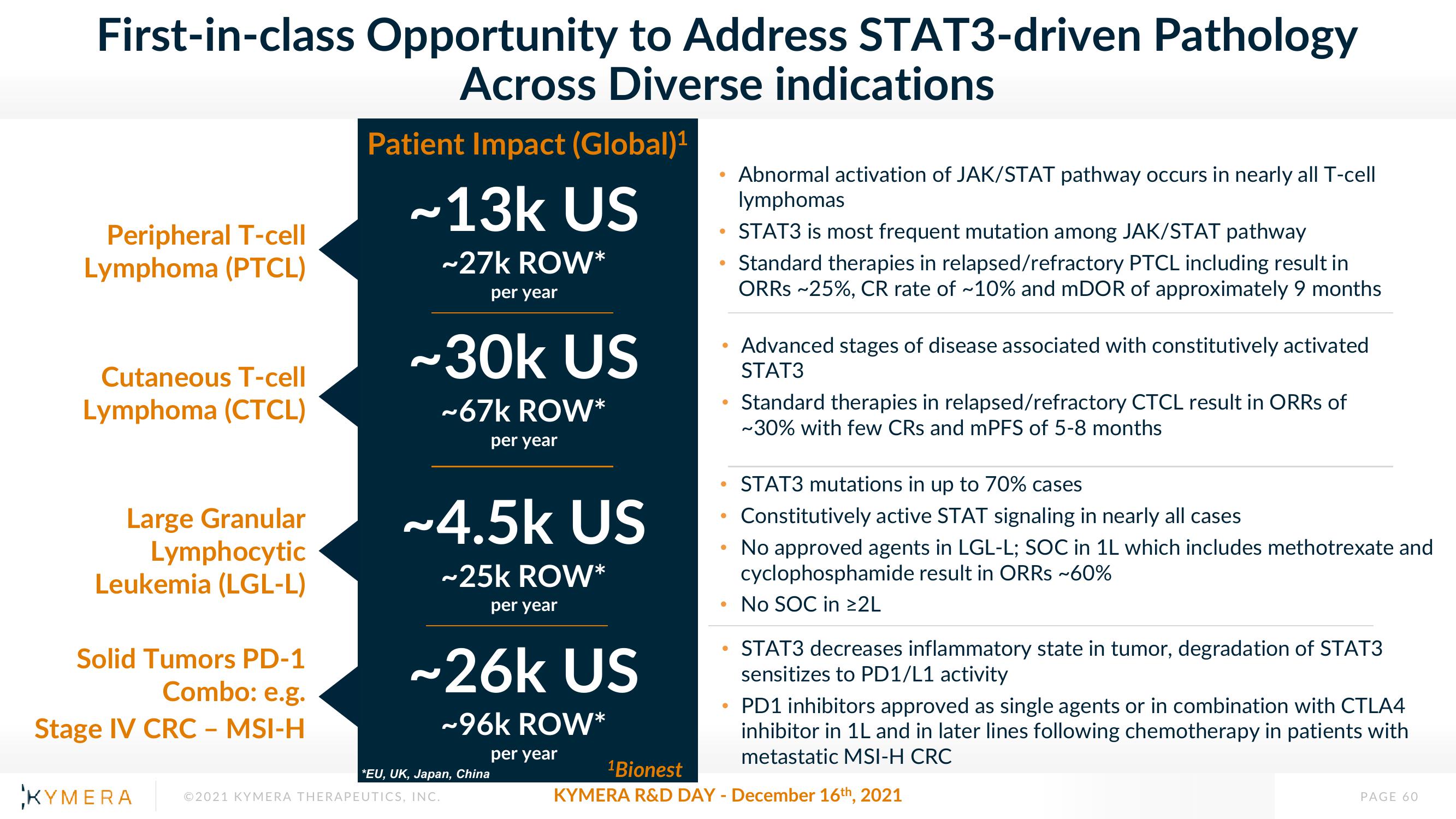
First-in-class Opportunity to Address STAT3-driven Pathology
Across Diverse indications
Peripheral T-cell
Lymphoma (PTCL)
Cutaneous T-cell
Lymphoma (CTCL)
Large Granular
Lymphocytic
Leukemia (LGL-L)
Solid Tumors PD-1
Combo: e.g.
Stage IV CRC - MSI-H
Patient Impact (Global)¹
~13k US
~27k ROW*
per year
~30k US
~67k ROW*
per year
~4.5k US
~25k ROW*
per year
~26k US
~96k ROW*
per year
*EU, UK, Japan, China
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
●
• STAT3 is most frequent mutation among JAK/STAT pathway
Standard therapies in relapsed/refractory PTCL including result in
ORRs ~25%, CR rate of ~10% and mDOR of approximately 9 months
●
●
Abnormal activation of JAK/STAT pathway occurs in nearly all T-cell
lymphomas
●
Advanced stages of disease associated with constitutively activated
STAT3
Standard therapies in relapsed/refractory CTCL result in ORRs of
~30% with few CRs and mPFS of 5-8 months
STAT3 mutations in up to 70% cases
Constitutively active STAT signaling in nearly all cases
No approved agents in LGL-L; SOC in 1L which includes methotrexate and
cyclophosphamide result in ORRs ~60%
No SOC in ≥2L
STAT3 decreases inflammatory state in tumor, degradation of STAT3
sensitizes to PD1/L1 activity
• PD1 inhibitors approved as single agents or in combination with CTLA4
inhibitor in 1L and in later lines following chemotherapy in patients with
metastatic MSI-H CRC
¹Bionest
KYMERA R&D DAY - December 16th, 2021
PAGE 60View entire presentation