AstraZeneca Results Presentation Deck
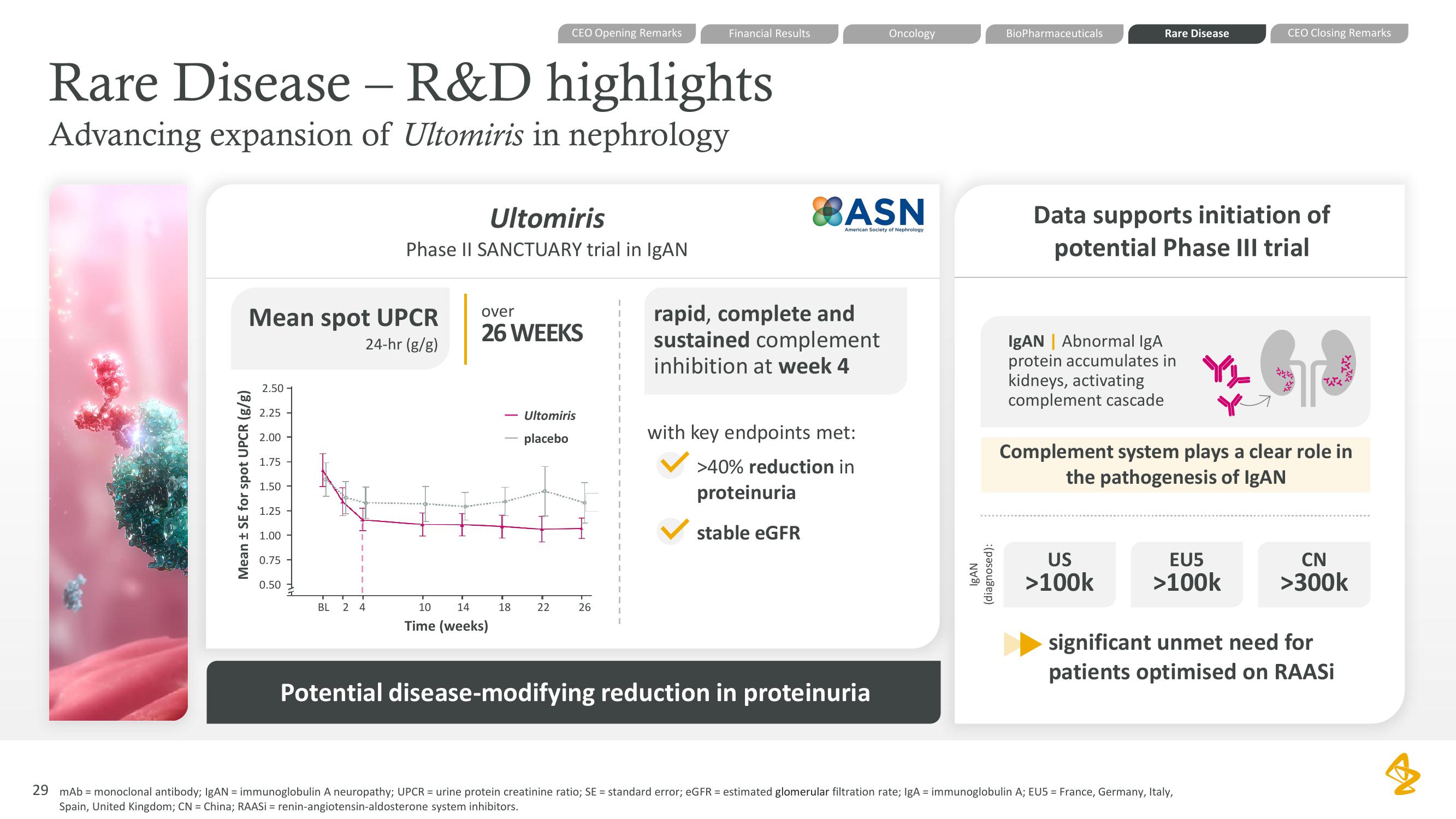
Rare Disease - R&D highlights
Advancing expansion of Ultomiris in nephrology
Mean spot UPCR
24-hr (g/g)
Mean ± SE for spot UPCR (g/g)
2.50
2.25
2.00
1.75
1.50
1.25
1.00
0.75
0.50
2
T
BL 2 4
Ultomiris
Phase II SANCTUARY trial in IgAN
CEO Opening Remarks
over
26 WEEKS
T
10
14
Time (weeks)
18
Ultomiris
placebo
22
26
Financial Results
1
rapid, complete and
sustained complement
inhibition at week 4
BASN
American Society of Nephrology
with key endpoints met:
>40% reduction in
proteinuria
stable eGFR
Oncology
Potential disease-modifying reduction in proteinuria
IgAN
(diagnosed):
BioPharmaceuticals
Rare Disease
Data supports initiation of
potential Phase III trial
IgAN | Abnormal IgA
protein accumulates in
kidneys, activating
complement cascade
US
>100k
CEO Closing Remarks
Complement system plays a clear role in
the pathogenesis of IgAN
EU5
>100k k >
TATL
29 mAb = monoclonal antibody; IgAN = immunoglobulin A neuropathy; UPCR = urine protein creatinine ratio; SE = standard error; eGFR = estimated glomerular filtration rate; IgA = immunoglobulin A; EU5 = France, Germany, Italy,
Spain, United Kingdom; CN = China; RAASI = renin-angiotensin-aldosterone system inhibitors.
CN
>300k
significant unmet need for
patients optimised on RAASIView entire presentation