Immix Biopharma Investor Presentation Deck
IMX-120 GLUT1 Biomarker-targeted for Inflammatory Bowel Disease
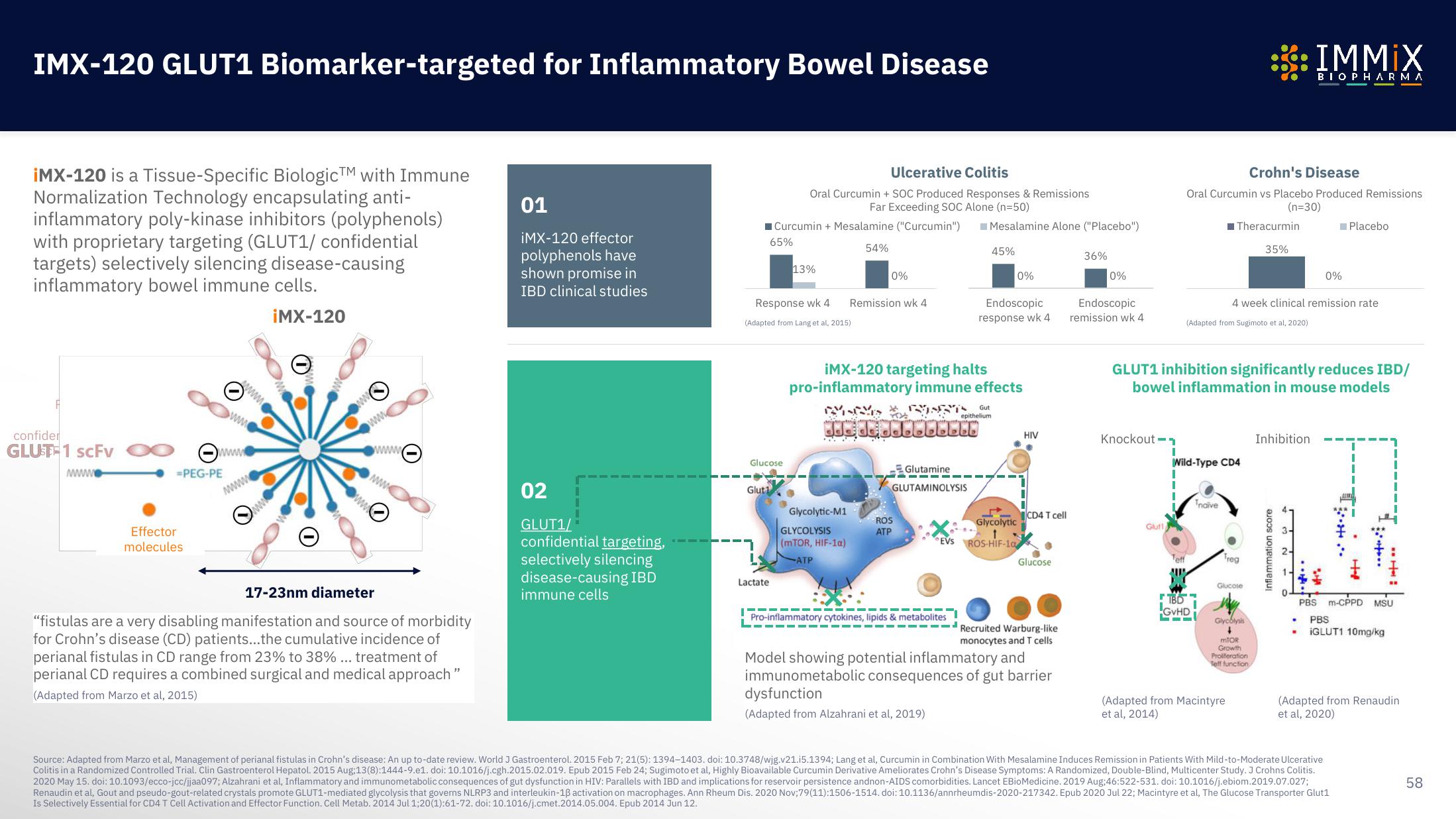
iMX-120 is a Tissue-Specific BiologicTM with Immune
Normalization Technology encapsulating anti-
inflammatory poly-kinase inhibitors (polyphenols)
with proprietary targeting (GLUT1/ confidential
targets) selectively silencing disease-causing
inflammatory bowel immune cells.
iMX-120
confider
GLUTF1 scFv
www.
w
-PEG-PE
Effector
molecules
www
ww
www.
www.
www.
www
www.
17-23nm diameter
"fistulas are a very disabling manifestation and source of morbidity
for Crohn's disease (CD) patients...the cumulative incidence of
perianal fistulas in CD range from 23% to 38% ... treatment of
perianal CD requires a combined surgical and medical approach"
(Adapted from Marzo et al, 2015)
01
iMX-120 effector
polyphenols have
shown promise in
IBD clinical studies
02
GLUT1/
confidential targeting,
selectively silencing
disease-causing IBD
immune cells
■Curcumin + Mesalamine ("Curcumin")
65%
Glucose
Ulcerative Colitis
Oral Curcumin + SOC Produced Responses & Remissions
Far Exceeding SOC Alone (n=50)
Response wk 4
(Adapted from Lang et al, 2015).
Glut1
Lactate
13%
54%
0%
Remission wk 4
Glycolytic-M1
GLYCOLYSIS
(mTOR, HIF-1a)
-ATP
مملوك مول تلك
iMX-120 targeting halts
pro-inflammatory immune effects
ROS
ATP
Glutamine
GLUTAMINOLYSIS
Mesalamine Alone ("Placebo")
Gut
58 epithelium
‒‒‒‒‒‒‒‒
45%
Endoscopic
response wk 4
Pro-inflammatory cytokines, lipids & metabolites
0%
Glycolytic
EVS ROS-HIF-1a
HIV
CD4 T cell
Glucose
2 Recruited Warburg-like
monocytes and T cells
Model showing potential inflammatory and
immunometabolic consequences of gut barrier
dysfunction
(Adapted from Alzahrani et al, 2019)
36%
0%
Endoscopic
remission wk 4
Knockout -
I
Glutl
Teff
AI
Crohn's Disease
Oral Curcumin vs Placebo Produced Remissions
(n=30)
(Adapted from Sugimoto et al, 2020)
Wild-Type CD4
1
TBD
GVHD
-
Theracurmin
35%
GLUT1 inhibition significantly reduces IBD/
bowel inflammation in mouse models
naive
●●●
S
Treg
4 week clinical remission rate
Glucose
(Adapted from Macintyre.
et al, 2014)
Glycolysis
4
MTOR
Growth
Proliferation
Teff function
IMMIX
BIOPHARMA
Inhibition
0%
Inflammation score
Placebo
+4
PBS m-CPPD MSU
PBS
IGLUT1 10mg/kg
Source: Adapted from Marzo et al, Management of perianal fistulas in Crohn's disease: An up to-date review. World J Gastroenterol. 2015 Feb 7; 21(5): 1394-1403. doi: 10.3748/wjg.v21.15.1394; Lang et al, Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative
Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2015 Aug;13(8):1444-9.e1. doi: 10.1016/j.cgh.2015.02.019. Epub 2015 Feb 24; Sugimoto et al, Highly Bioavailable Curcumin Derivative Ameliorates Crohn's Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J Crohns Colitis.
2020 May 15. doi: 10.1093/ecco-jcc/jjaa097; Alzahrani et al, Inflammatory and immunometabolic consequences of gut dysfunction in HIV: Parallels with IBD and implications for reservoir persistence andnon-AIDS comorbidities. Lancet EBioMedicine. 2019 Aug;46:522-531. doi: 10.1016/j.ebiom.2019.07.027;
Renaudin et al, Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-18 activation on macrophages. Ann Rheum Dis. 2020 Nov;79(11):1506-1514. doi: 10.1136/annrheumdis-2020-217342. Epub 2020 Jul 22; Macintyre et al, The Glucose Transporter Glut1
Is Selectively Essential for CD4 T Cell Activation and Effector Function. Cell Metab. 2014 Jul 1;20(1):61-72. doi: 10.1016/j.cmet.2014.05.004. Epub 2014 Jun 12.
..Hei:
(Adapted from Renaudin
et al, 2020)
58View entire presentation