Genelux Investor Presentation Deck
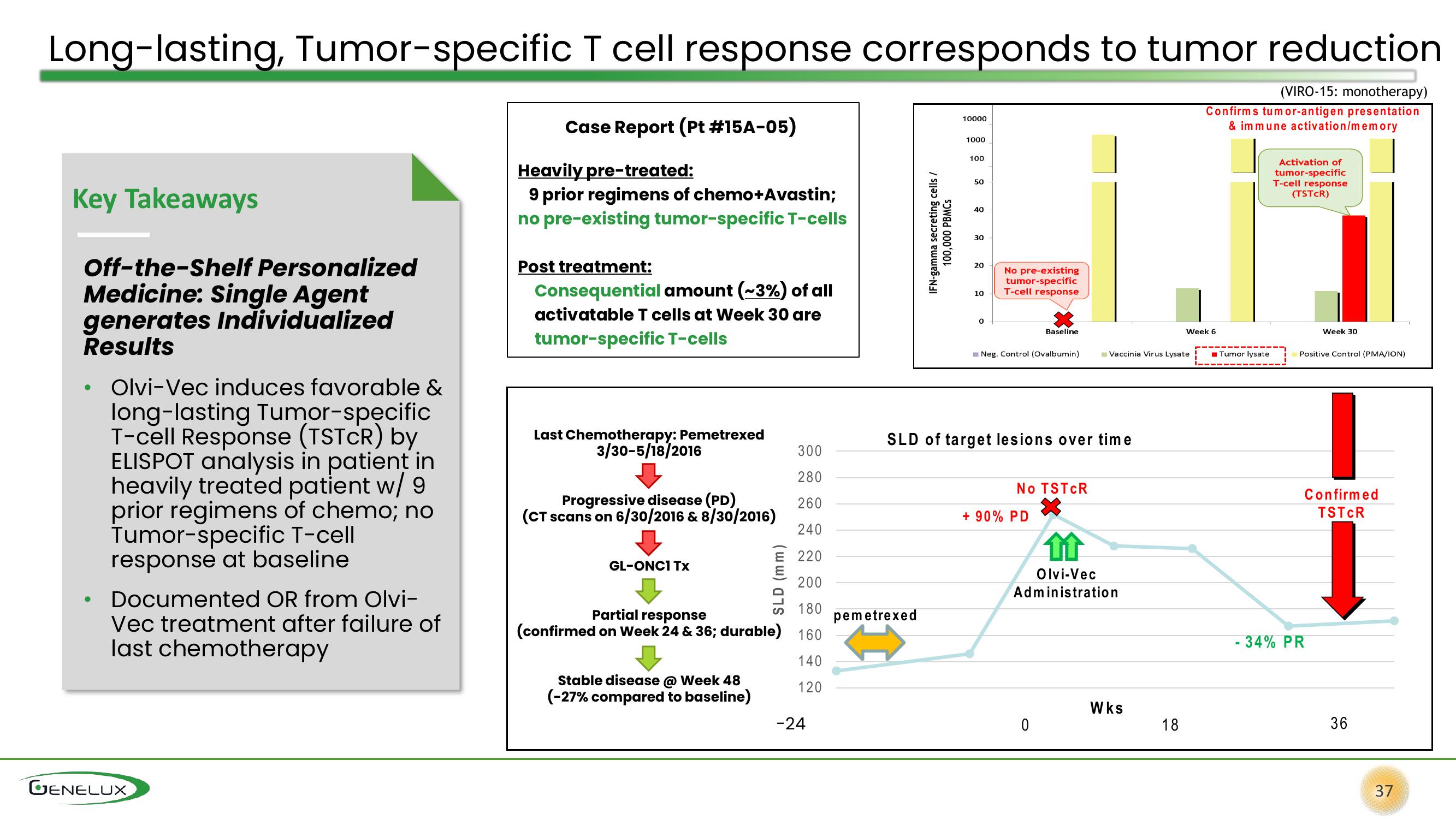
Long-lasting, Tumor-specific T cell response corresponds to tumor reduction
(VIRO-15: monotherapy)
Confirms tumor-antigen presentation
& immune activation/memory
Key Takeaways
Off-the-Shelf Personalized
Medicine: Single Agent
generates Individualized
Results
• Olvi-Vec induces favorable &
long-lasting Tumor-specific
T-cell Response (TSTCR) by
ELISPOT analysis in patient in
heavily treated patient w/ 9
prior regimens of chemo; no
Tumor-specific T-cell
response at baseline
●
Documented OR from Olvi-
Vec treatment after failure of
last chemotherapy
GENELUX
Case Report (Pt #15A-05)
Heavily pre-treated:
9 prior regimens of chemo+Avastin;
no pre-existing tumor-specific T-cells
Post treatment:
Consequential amount (~3%) of all
activatable T cells at Week 30 are
tumor-specific T-cells
Last Chemotherapy: Pemetrexed
3/30-5/18/2016
300
280
260
240
220
200
180
Partial response
(confirmed on Week 24 & 36; durable) 160
140
120
Progressive disease (PD)
(CT scans on 6/30/2016 & 8/30/2016)
GL-ONC1 Tx
Stable disease @ Week 48
(-27% compared to baseline)
SLD (mm)
-24
IFN-gamma secreting cells /
100,000 PBMCs
pemetrexed
10000
1000
100
50
40
30
20
10
0
No pre-existing
tumor-specific
T-cell response
Neg. Control (Ovalbumin)
Baseline
SLD of target lesions over time
No TSTCR
+ 90% PD
0
Vaccinia Virus Lysate
价
Olvi-Vec
Administration
Wks
Week 6
18
Tumor lysate
Activation of
tumor-specific
T-cell response
(TSTCR)
Week 30
Positive Control (PMA/ION)
1
Confirmed
TSTCR
- 34% PR
36
37View entire presentation