Kymera Investor Presentation Deck
●
DLX
●
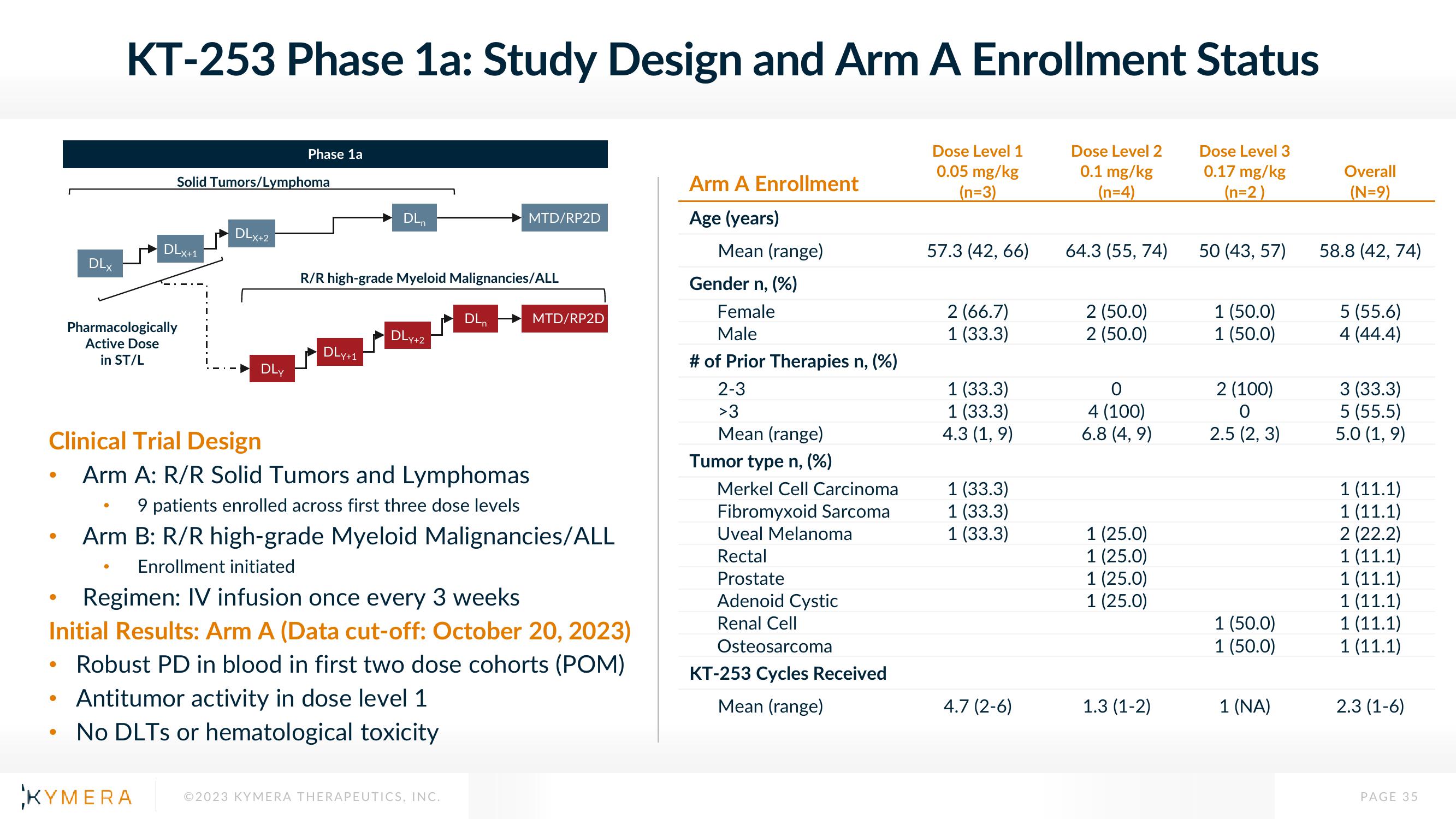
KT-253 Phase 1a: Study Design and Arm A Enrollment Status
Dose Level 1
0.05 mg/kg
(n=3)
Dose Level 2
0.1 mg/kg
(n=4)
Dose Level 3
0.17 mg/kg
(n=2)
●
Solid Tumors/Lymphoma
DLXH1
Pharmacologically
Active Dose
in ST/L
DLxt2
Phase 1a
DLY
DLn
DLy+1
R/R high-grade Myeloid Malignancies/ALL
DLY +2
Clinical Trial Design
Arm A: R/R Solid Tumors and Lymphomas
9 patients enrolled across first three dose levels
Arm B: R/R high-grade Myeloid Malignancies/ALL
Enrollment initiated
MTD/RP2D
DLn
• Antitumor activity in dose level 1
• No DLTs or hematological toxicity
Regimen: IV infusion once every 3 weeks
Initial Results: Arm A (Data cut-off: October 20, 2023)
• Robust PD in blood in first two dose cohorts (POM)
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
MTD/RP2D
Arm A Enrollment
Age (years)
Mean (range)
Gender n, (%)
Female
Male
# of Prior Therapies n, (%)
2-3
>3
Mean (range)
Tumor type n, (%)
Merkel Cell Carcinoma
Fibromyxoid Sarcoma
Uveal Melanoma
Rectal
Prostate
Adenoid Cystic
Renal Cell
Osteosarcoma
KT-253 Cycles Received
Mean (range)
57.3 (42, 66)
2 (66.7)
1 (33.3)
1 (33.3)
1 (33.3)
4.3 (1,9)
1 (33.3)
1 (33.3)
1 (33.3)
4.7 (2-6)
64.3 (55, 74)
2 (50.0)
2 (50.0)
O
4 (100)
6.8 (4, 9)
1 (25.0)
1 (25.0)
1 (25.0)
1 (25.0)
1.3 (1-2)
50 (43, 57)
1 (50.0)
1 (50.0)
2 (100)
O
2.5 (2, 3)
1 (50.0)
1 (50.0)
1 (NA)
Overall
(N=9)
58.8 (42,74)
5 (55.6)
4 (44.4)
3 (33.3)
5 (55.5)
5.0 (1,9)
1 (11.1)
1 (11.1)
2 (22.2)
1 (11.1)
1 (11.1)
1 (11.1)
1 (11.1)
1 (11.1)
2.3 (1-6)
PAGE 35View entire presentation