Immix Biopharma Investor Presentation Deck
NXC-201 Clinical Results Demonstrate Potential for Best-in-Class Efficacy & Safety
in Multiple Myeloma, a $29 billion market by 2027
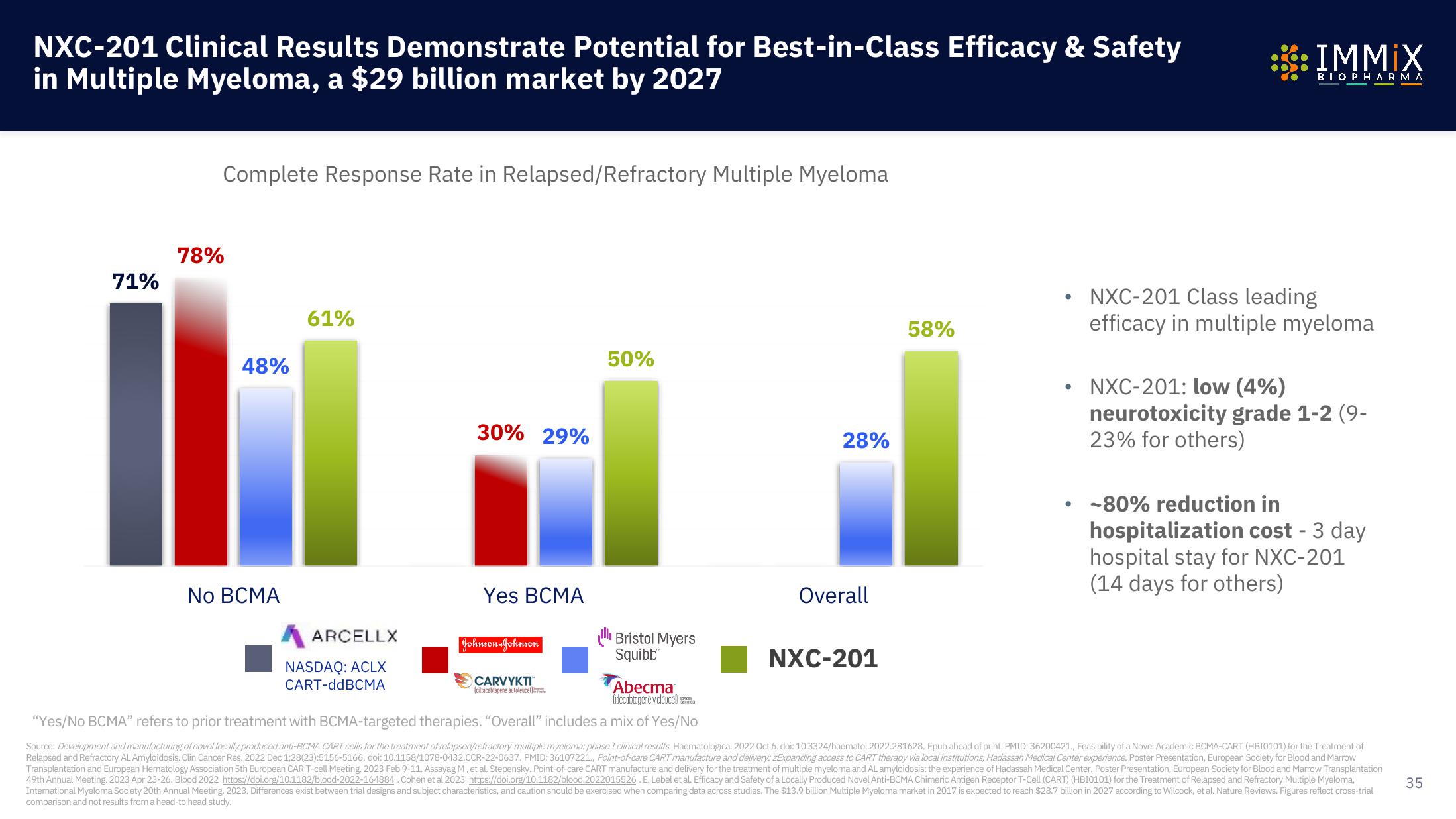
71%
Complete Response Rate in Relapsed/Refractory Multiple Myeloma
78%
48%
No BCMA
61%
ARCELLX
NASDAQ: ACLX
CART-ddBCMA
30% 29%
Yes BCMA
Johnson & Johnson
CARVYKTI
Iciltacabtagene autoleucell
50%
Bristol Myers
Squibb™
28%
Overall
NXC-201
58%
●
●
●●●
S
IMMIX
BIOPHARMA
NXC-201 Class leading
efficacy in multiple myeloma
NXC-201: low (4%)
neurotoxicity grade 1-2 (9-
23% for others)
• -80% reduction in
hospitalization cost - 3 day
hospital stay for NXC-201
(14 days for others)
Abecma
lidecabtagene vicleuce)
"Yes/No BCMA" refers to prior treatment with BCMA-targeted therapies. "Overall" includes a mix of Yes/No
Source: Development and manufacturing of novel locally produced anti-BCMA CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica. 2022 Oct 6. doi: 10.3324/haematol.2022.281628. Epub ahead of print. PMID: 36200421., Feasibility of a Novel Academic BCMA-CART (HBI0101) for the Treatment of
Relapsed and Refractory AL Amyloidosis. Clin Cancer Res. 2022 Dec 1;28(23):5156-5166. doi: 10.1158/1078-0432.CCR-22-0637. PMID: 36107221., Point-of-care CART manufacture and delivery: zExpanding access to CART therapy via local institutions, Hadassah Medical Center experience. Poster Presentation, European Society for Blood and Marrow
Transplantation and European Hematology Association 5th European CAR T-cell Meeting, 2023 Feb 9-11. Assayag M, et al. Stepensky. Point-of-care CART manufacture and delivery for the treatment of multiple myeloma and AL amyloidosis: the experience of Hadassah Medical Center. Poster Presentation, European Society for Blood and Marrow Transplantation
49th Annual Meeting. 2023 Apr 23-26. Blood 2022 https://doi.org/10.1182/blood-2022-164884. Cohen et al 2023 https://doi.org/10.1182/blood.2022015526. E. Lebel et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma,
International Myeloma Society 20th Annual Meeting. 2023. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies. The $13.9 billion Multiple Myeloma market in 2017 is expected to reach $28.7 billion in 2027 according to Wilcock, et al. Nature Reviews. Figures reflect cross-trial
comparison and not results from a head-to head study..
35View entire presentation