MaxCyte IPO Presentation Deck
Ability to Drive Complex Engineering Via Non-Viral Delivery for
CRISPR-Engineered T Cells in Patients with Refractory Cancer
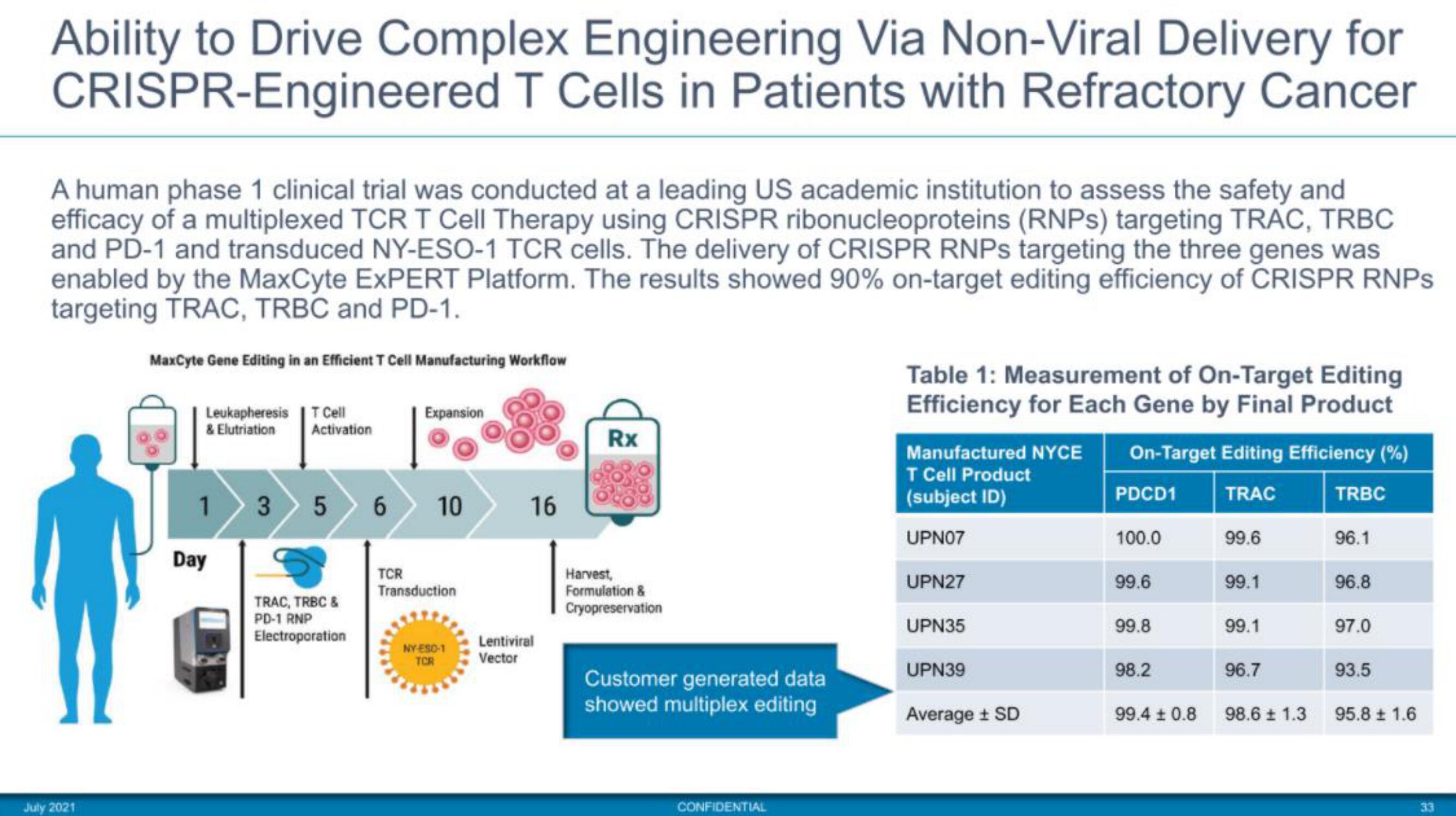
A human phase 1 clinical trial was conducted at a leading US academic institution to assess the safety and
efficacy of a multiplexed TCR T Cell Therapy using CRISPR ribonucleoproteins (RNPS) targeting TRAC, TRBC
and PD-1 and transduced NY-ESO-1 TCR cells. The delivery of CRISPR RNPS targeting the three genes was
enabled by the MaxCyte EXPERT Platform. The results showed 90% on-target editing efficiency of CRISPR RNPs
targeting TRAC, TRBC and PD-1.
July 2021
MaxCyte Gene Editing in an Efficient T Cell Manufacturing Workflow
Leukapheresis | T Cell
& Elutriation
Day
Activation
3 5
TRAC, TRBC &
PD-1 RNP
Electroporation
6
Expansion
10
TCR
Transduction
NY-ESO-1
TCR
16
Lentiviral
Vector
Rx
Harvest,
Formulation &
Cryopreservation
Customer generated data
showed multiplex editing
CONFIDENTIAL
Table 1: Measurement of On-Target Editing
Efficiency for Each Gene by Final Product
On-Target Editing Efficiency (%)
TRAC
TRBC
96.1
96.8
97.0
Manufactured NYCE
T Cell Product
(subject ID)
UPN07
UPN27
UPN35
UPN39
Average ± SD
PDCD1
100.0
99.6
99.8
98.2
99.4 ± 0.8
99.6
99.1
99.1
96.7
98.6 ± 1.3
93.5
95.8 ± 1.6
33View entire presentation