AstraZeneca Investor Day Presentation Deck
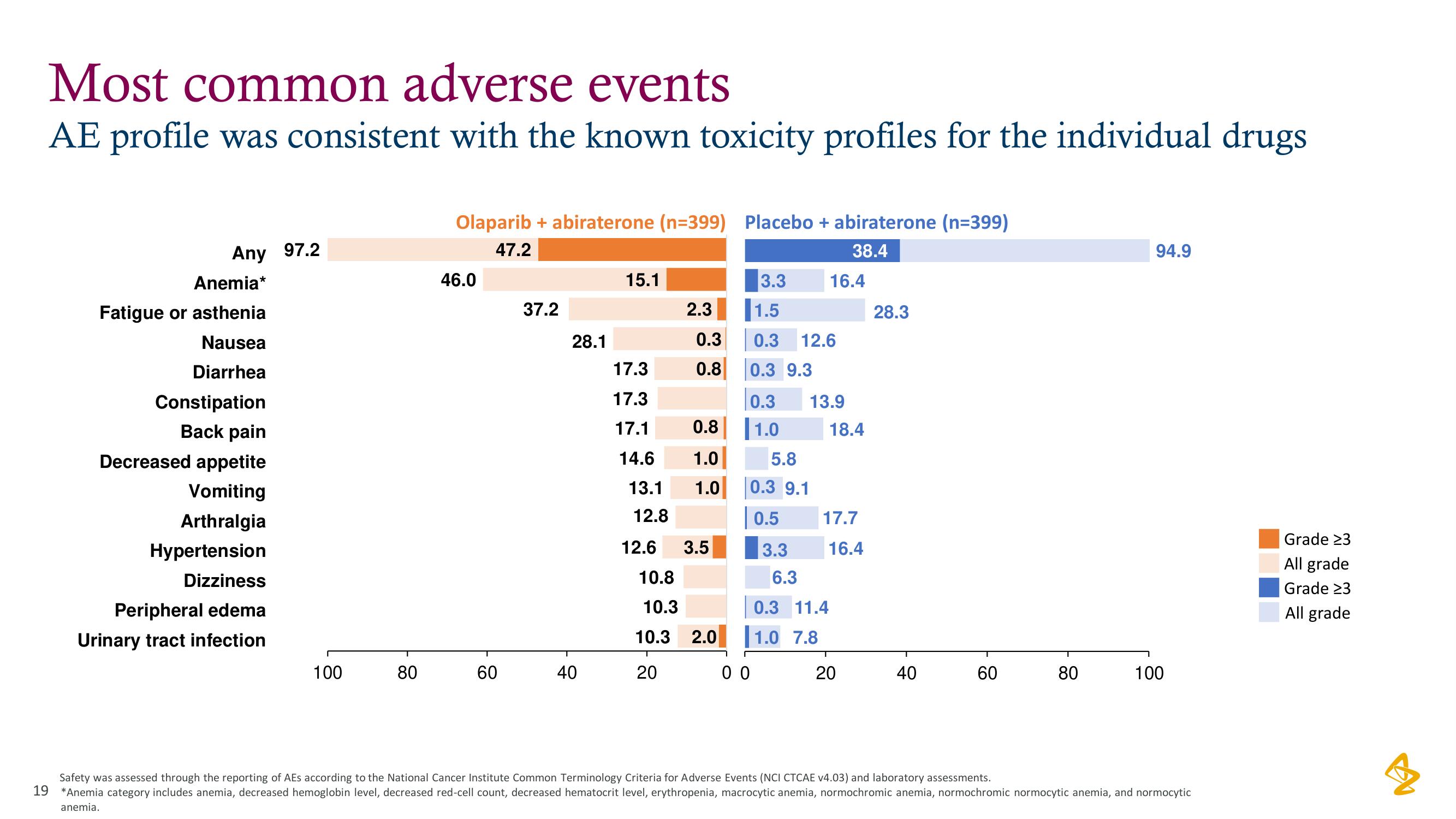
Most common adverse events
AE profile was consistent with the known toxicity profiles for the individual drugs
Any 97.2
Anemia*
Fatigue or asthenia
Nausea
Diarrhea
Constipation
Back pain
Decreased appetite
Vomiting
Arthralgia
Hypertension
Dizziness
Peripheral edema
Urinary tract infection
100
80
Olaparib + abiraterone (n=399) Placebo + abiraterone (n=399)
47.2
38.4
46.0
60
37.2
28.1
40
15.1
17.3
17.3
17.1
14.6
13.1
0.8
1.0
1.0
2.3
1.5
0.3 0.3 12.6
0.8 0.3 9.3
12.8
12.6 3.5
10.8
10.3
10.3 2.0
20
3.3
0.3
00
1.0
3.3
16.4
6.3
13.9
5.8
0.3 9.1
0.5 17.7
16.4
0.3 11.4
1.0 7.8
18.4
20
28.3
40
60
80
94.9
100
Safety was assessed through the reporting of AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v4.03) and laboratory assessments.
19 *Anemia category includes anemia, decreased hemoglobin level, decreased red-cell count, decreased hematocrit level, erythropenia, macrocytic anemia, normochromic anemia,normochromic normocytic anemia, and normocytic
anemia.
Grade 23
All grade
Grade 23
All grade
3View entire presentation