Imara M&A
BLI (photons/sx106)
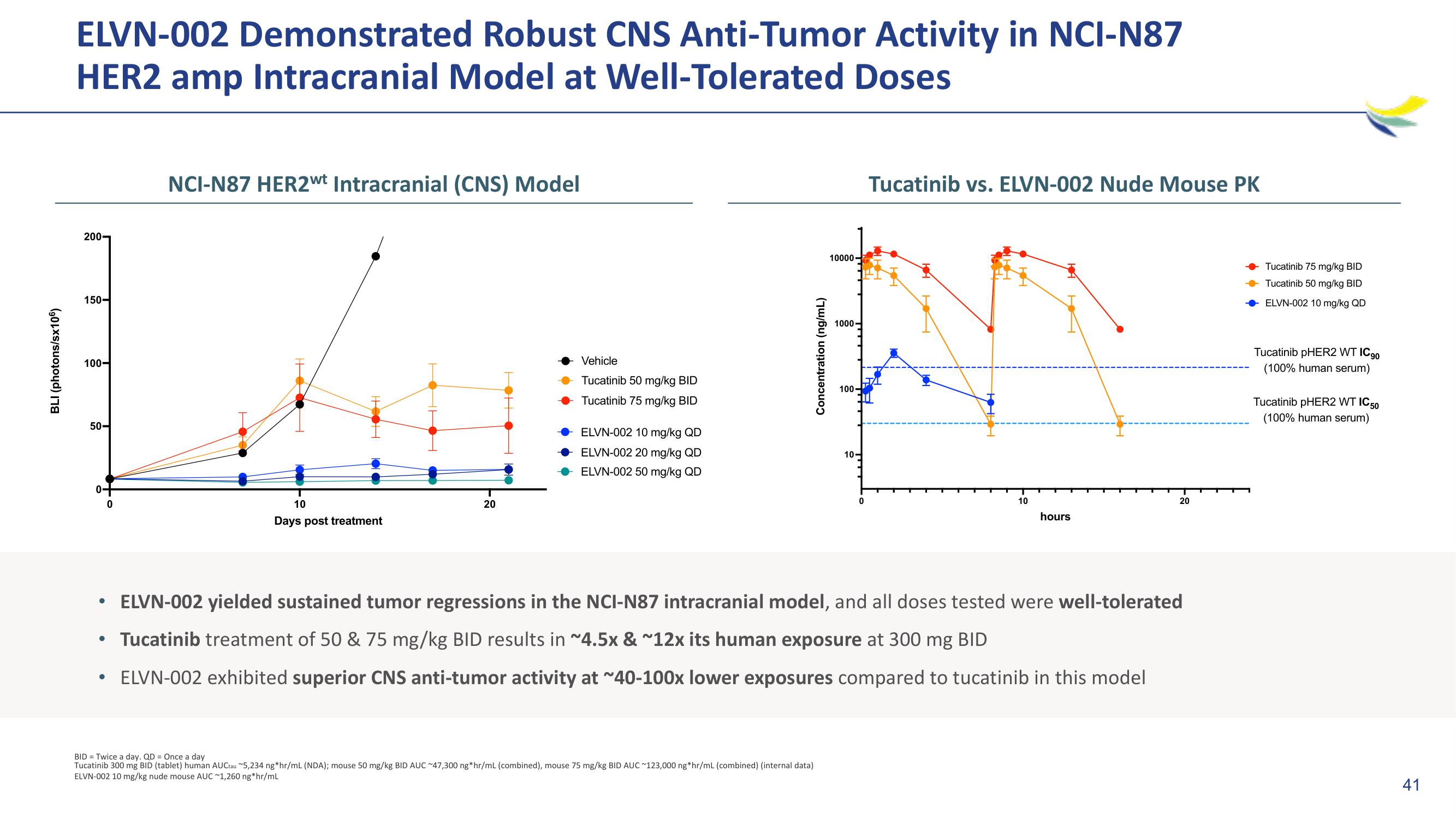
ELVN-002 Demonstrated Robust CNS Anti-Tumor Activity in NCI-N87
HER2 amp Intracranial Model at Well-Tolerated Doses
200-
150-
100-
50-
●
●
0
NCI-N87 HER2wt Intracranial (CNS) Model
H
HOCHIL
10
Days post treatment
20
HH100
Vehicle
Tucatinib 50 mg/kg BID
Tucatinib 75 mg/kg BID
ELVN-002 10 mg/kg QD
ELVN-002 20 mg/kg QD
ELVN-002 50 mg/kg QD
Concentration (ng/mL)
BID = Twice a day. QD = Once a day
Tucatinib 300 mg BID (tablet) human AUCtau ~5,234 ng*hr/mL (NDA); mouse 50 mg/kg BID AUC ~47,300 ng*hr/mL (combined), mouse 75 mg/kg BID AUC ~123,000 ng*hr/mL (combined) (internal data)
ELVN-002 10 mg/kg nude mouse AUC ~1,260 ng*hr/mL
10000-
1000-
100-
10-
0
Tucatinib vs. ELVN-002 Nude Mouse PK
10
hours
20
ELVN-002 yielded sustained tumor regressions in the NCI-N87 intracranial model, and all doses tested were well-tolerated
Tucatinib treatment of 50 & 75 mg/kg BID results in ~4.5x & ~12x its human exposure at 300 mg BID
ELVN-002 exhibited superior CNS anti-tumor activity at ~40-100x lower exposures compared to tucatinib in this model
Tucatinib 75 mg/kg BID
Tucatinib 50 mg/kg BID
ELVN-002 10 mg/kg QD
Tucatinib pHER2 WT IC90
(100% human serum)
Tucatinib pHER2 WT IC50
(100% human serum)
41View entire presentation