Aravive Investor Presentation Deck
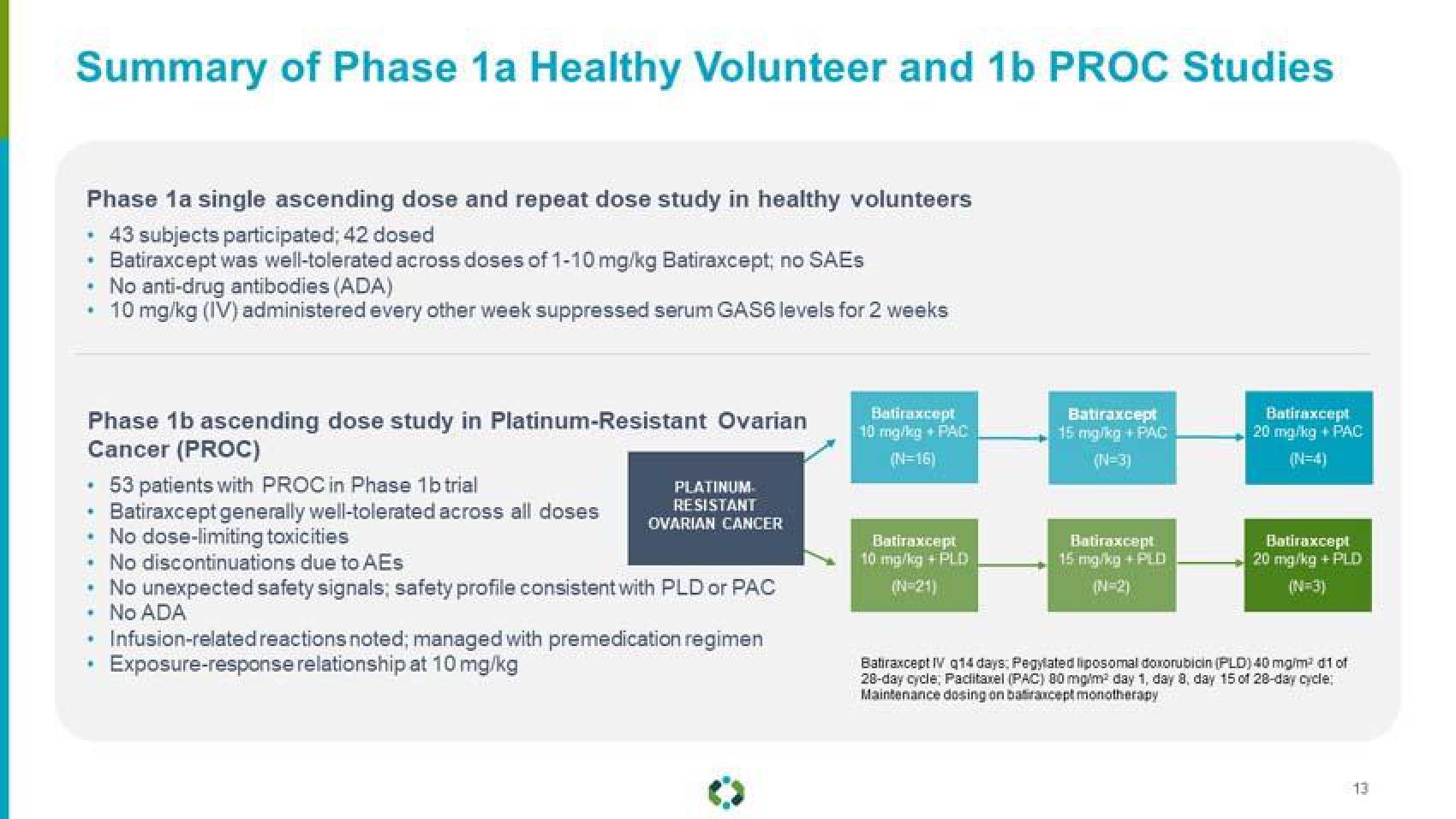
Summary of Phase 1a Healthy Volunteer and 1b PROC Studies
Phase 1a single ascending dose and repeat dose study in healthy volunteers
• 43 subjects participated; 42 dosed
Batiraxcept was well-tolerated across doses of 1-10 mg/kg Batiraxcept; no SAEs
Phase 1b ascending dose study in Platinum-Resistant Ovarian
Cancer (PROC)
No anti-drug antibodies (ADA)
10 mg/kg (IV) administered every other week suppressed serum GAS6 levels for 2 weeks
• 53 patients with PROC in Phase 1b trial
Batiraxcept generally well-tolerated across all doses
• No dose-limiting toxicities
• No discontinuations due to AEs
i
• No unexpected safety signals; safety profile consistent with PLD or PAC
No ADA
#
PLATINUM
RESISTANT
OVARIAN CANCER
Infusion-related reactions noted; managed with premedication regimen
Exposure-response relationship at 10 mg/kg
Batiraxcept
10 mg/kg + PAC
(N=16)
Batiraxcept
10 mg/kg + PLD
Batiraxcept
15 mg/kg + PAC
(N=3)
Batiraxcept
15 mg/kg + PLD
(N=2)
Batiraxcept
20 mg/kg + PAC
Batiraxcept
20 mg/kg + PLD
(N=3)
Batiraxcept IV q14 days: Pegylated liposomal doxorubicin (PLD) 40 mg/m² d1 of
28-day cycle, Paclitaxel (PAC) 80 mg/m² day 1, day 8, day 15 of 28-day cycle:
Maintenance dosing on batraxcept monotherapy
13View entire presentation