Certara Investor Day Presentation Deck
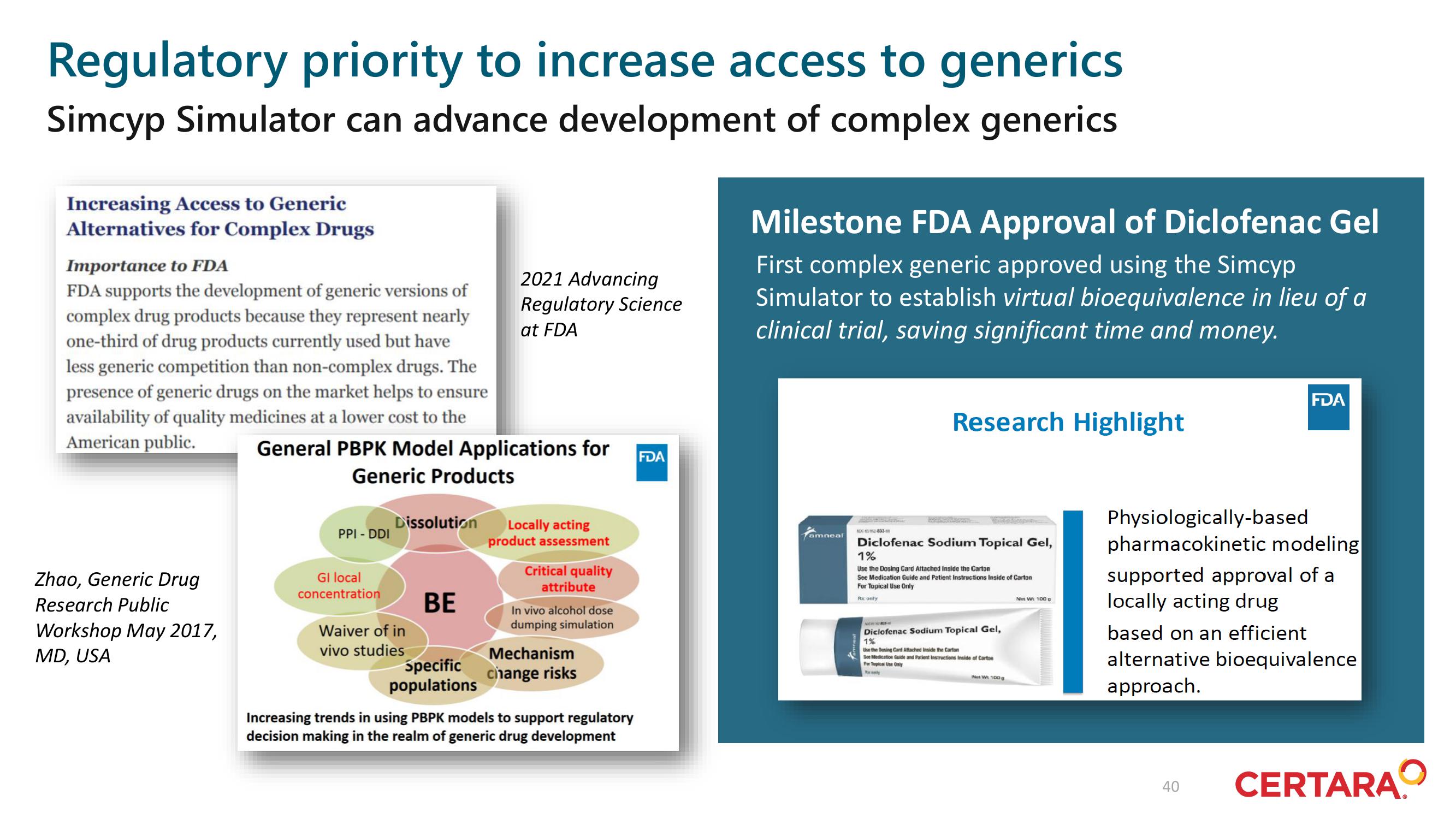
Regulatory priority to increase access to generics
Simcyp Simulator can advance development of complex generics
Increasing Access to Generic
Alternatives for Complex Drugs
Importance to FDA
FDA supports the development of generic versions of
complex drug products because they represent nearly
one-third of drug products currently used but have
less generic competition than non-complex drugs. The
presence of generic drugs on the market helps to ensure
availability of quality medicines at a lower cost to the
American public.
Zhao, Generic Drug
Research Public
Workshop May 2017,
MD, USA
General PBPK Model Applications for
Generic Products
PPI - DDI
Gl local
concentration
Dissolution
Waiver of in
vivo studies
BE
2021 Advancing
Regulatory Science
at FDA
Specific
populations
Locally acting
product assessment
Critical quality
attribute
In vivo alcohol dose
dumping simulation
Mechanism
change risks
Increasing trends in using PBPK models to support regulatory
decision making in the realm of generic drug development
FDA
Milestone FDA Approval of Diclofenac Gel
First complex generic approved using the Simcyp
Simulator to establish virtual bioequivalence in lieu of a
clinical trial, saving significant time and money.
7amneal
10000162-833-
air
Research Highlight
THEO COMPAR
Diclofenac Sodium Topical Gel,
1%
Use the Dosing Card Attached Inside the Carton
See Medication Guide and Patient Instructions Inside of Carton
For Topical Use Only
Rx only
Diclofenac Sodium Topical Gel,
1%
Use the Dosing Card Attached Inside the Carton
See Medication Guide and Patient Instructions Inside of Carton
For Topical Use Only
Reply
Net Wt 100 g
Net Wt 100 g
FDA
Physiologically-based
pharmacokinetic modeling
supported approval of a
locally acting drug
based on an efficient
alternative bioequivalence
approach.
40
CERTARAView entire presentation