MaxCyte Investor Presentation Deck
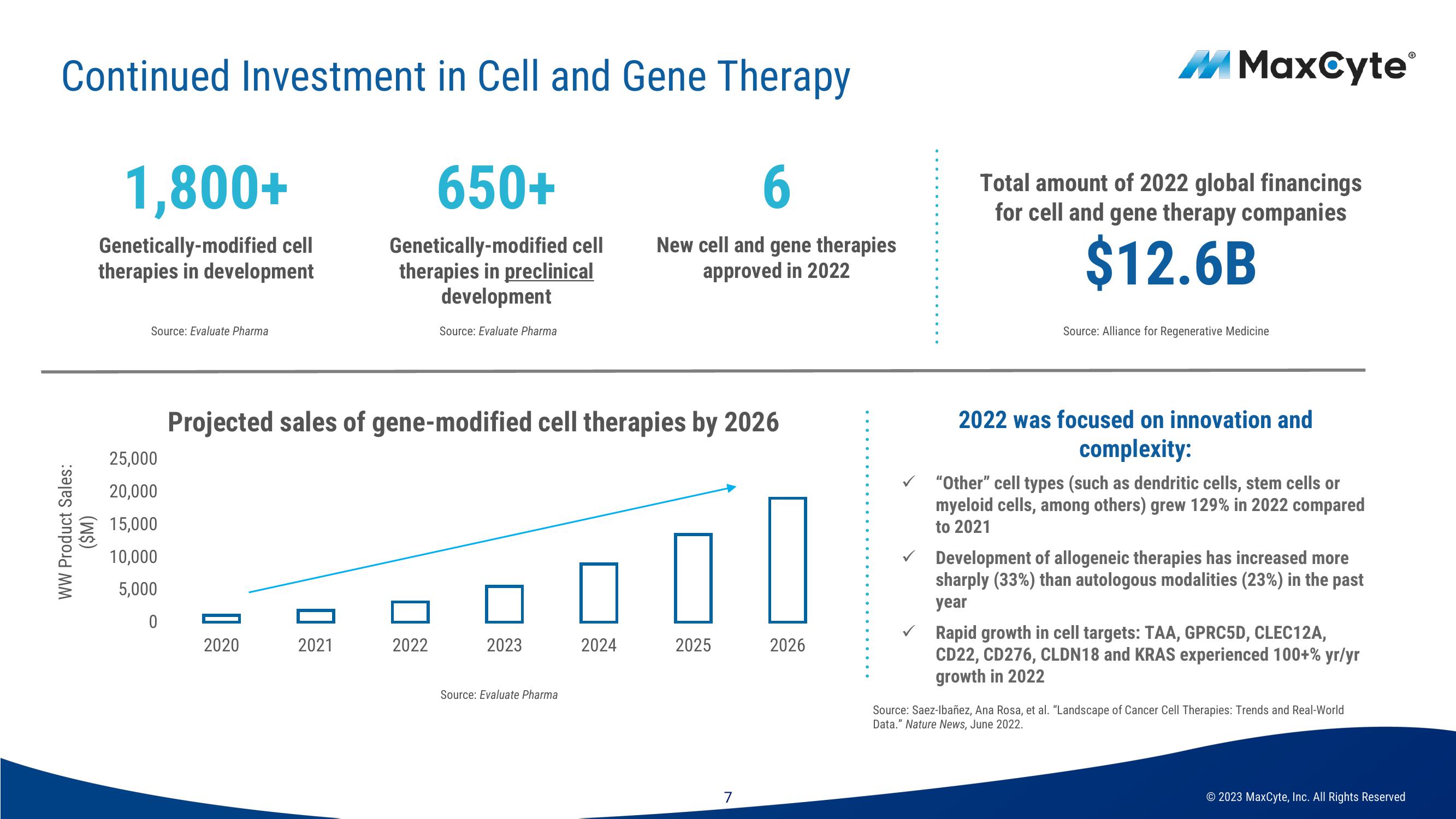
Continued Investment in Cell and Gene Therapy
650+
6
Genetically-modified cell
therapies in preclinical
development
New cell and gene therapies
approved in 2022
WW Product Sales:
($M)
1,800+
Genetically-modified cell
therapies in development
Source: Evaluate Pharma
25,000
20,000
15,000
10,000
5,000
0
Projected sales of gene-modified cell therapies by 2026
2020
0
2021
Source: Evaluate Pharma
2022
2023
Source: Evaluate Pharma
2024
2025
2026
M MaxCyte
Total amount of 2022 global financings
for cell and gene therapy companies
$12.6B
Source: Alliance for Regenerative Medicine
2022 was focused on innovation and
complexity:
"Other" cell types (such as dendritic cells, stem cells or
myeloid cells, among others) grew 129% in 2022 compared
to 2021
Development of allogeneic therapies has increased more
sharply (33%) than autologous modalities (23%) in the past
year
Rapid growth in cell targets: TAA, GPRC5D, CLEC12A,
CD22, CD276, CLDN18 and KRAS experienced 100+% yr/yr
growth in 2022
Source: Saez-Ibañez, Ana Rosa, et al. "Landscape of Cancer Cell Therapies: Trends and Real-World
Data." Nature News, June 2022.
© 2023 MaxCyte, Inc. All Rights ReservedView entire presentation