Ocuphire Pharma Investor Updates
P
19
Percent of Subjects (%)
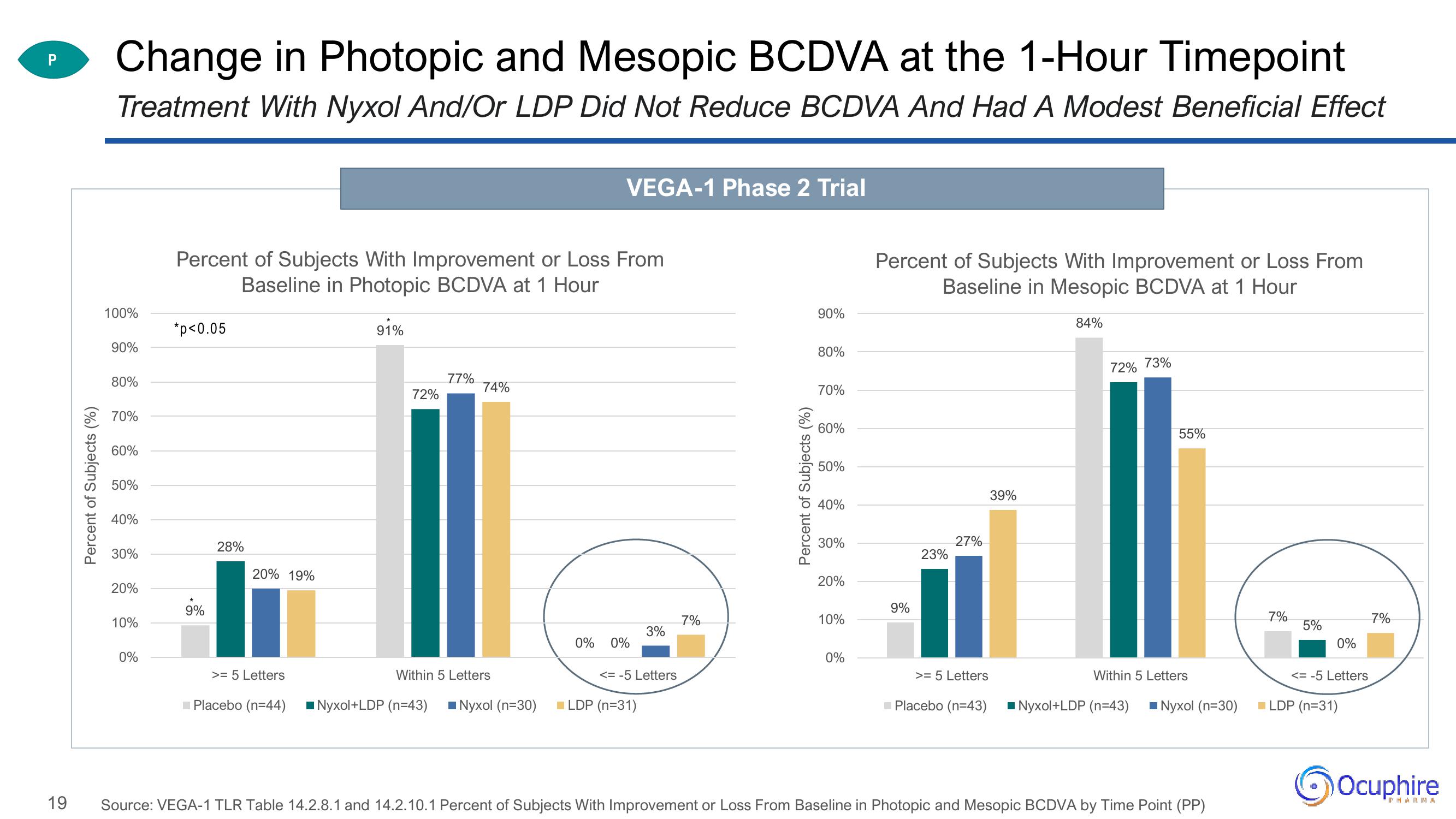
Change in Photopic and Mesopic BCDVA at the 1-Hour Timepoint
Treatment With Nyxol And/Or LDP Did Not Reduce BCDVA And Had A Modest Beneficial Effect
100%
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
Percent of Subjects With Improvement or Loss From
Baseline in Photopic BCDVA at 1 Hour
91%
*p<0.05
9%
28%
20% 19%
72%
77%
74%
>= 5 Letters
Placebo (n=44) Nyxol+LDP (n=43) Nyxol (n=30)
Within 5 Letters
VEGA-1 Phase 2 Trial
0%
0%
3%
<= -5 Letters
LDP (n=31)
7%
Percent of Subjects (%)
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
Percent of Subjects With Improvement or Loss From
Baseline in Mesopic BCDVA at 1 Hour
84%
9%
23%
27%
39%
>= 5 Letters
Placebo (n=43)
72% 73%
55%
Within 5 Letters
Nyxol+LDP (n=43)
Nyxol (n=30)
Source: VEGA-1 TLR Table 14.2.8.1 and 14.2.10.1 Percent of Subjects With Improvement or Loss From Baseline in Photopic and Mesopic BCDVA by Time Point (PP)
7%
5%
0%
<= -5 Letters
LDP (n=31)
7%
Ocuphire
PHARMAView entire presentation