BioNTech Investor Day Presentation Deck
iNeST I Autogene cevumeran (BNT122)
Phase 1 as monotherapy and in combination with atezolizumab
●
●
●
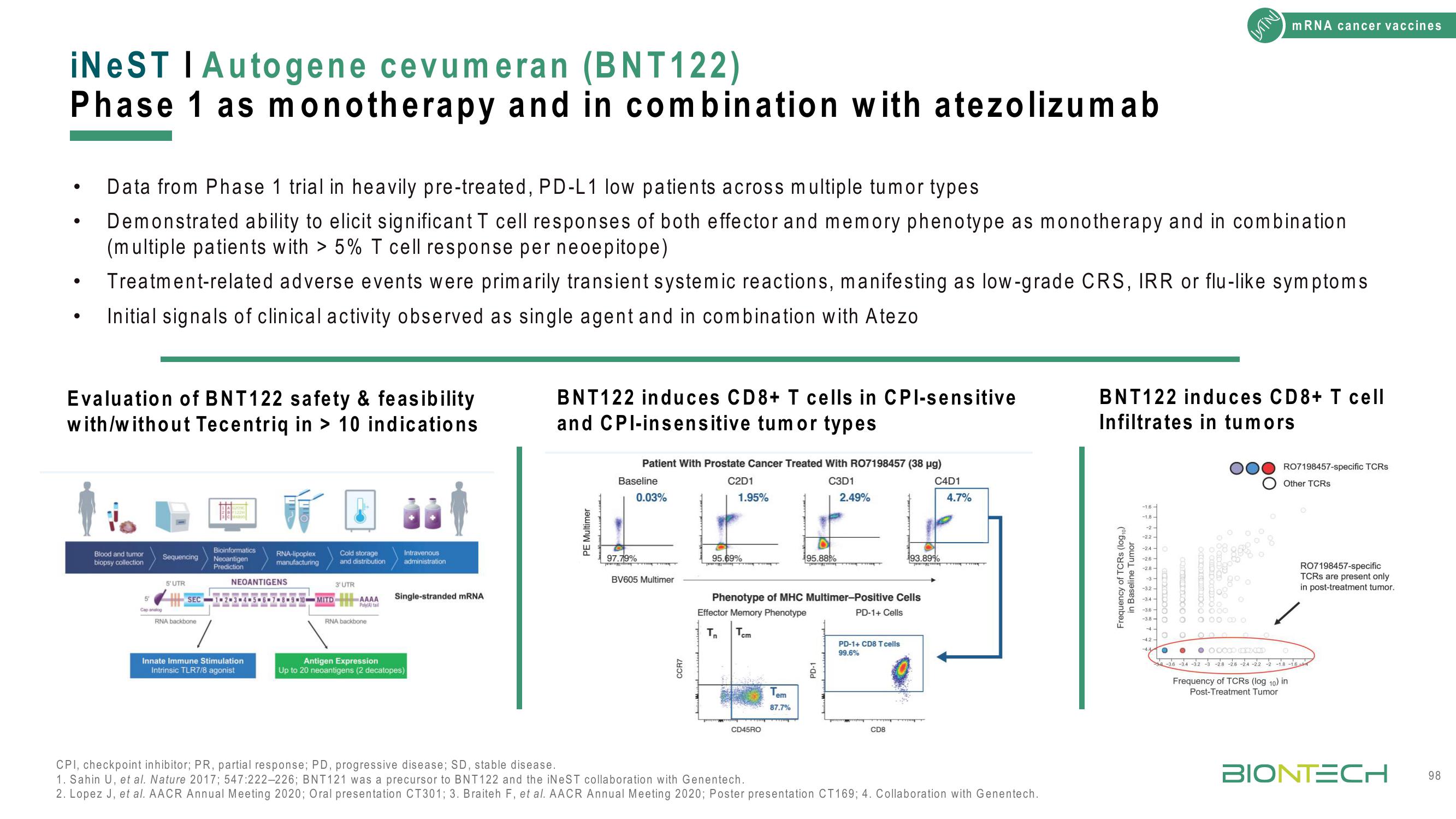
Evaluation of BNT122 safety & feasibility
with/without Tecentriq in> 10 indications
Data from Phase 1 trial in heavily pre-treated, PD-L1 low patients across multiple tumor types
Demonstrated ability to elicit significant T cell responses of both effector and memory phenotype as monotherapy and in combination
(multiple patients with > 5% T cell response per neoepitope)
Treatment-related adverse events were primarily transient systemic reactions, manifesting as low-grade CRS, IRR or flu-like symptoms
Initial signals of clinical activity observed as single agent and in combination with Atezo
Blood and tumor
biopsy collection
5
Cap analog
Sequencing
5' UTR
SEC
RNA backbone
Bioinformatics
Neoantigen
Prediction
NEOANTIGENS
2-3-4-5
RNA-lipoplex
manufacturing
Innate Immune Stimulation
Intrinsic TLR7/8 agonist
Cold storage
and distribution
3' UTR
MITDAAAA
RNA backbone
Intravenous
administration
Single-stranded mRNA
Antigen Expression
Up to 20 neoantigens (2 decatopes)
BNT122 induces CD8+ T cells in CPI-sensitive
and CPI-insensitive tumor types
PE Multimer
Patient with Prostate Cancer Treated With RO7198457 (38 µg)
C2D1
C3D1
1.95%
2.49%
Baseline
0.03%
97.79%
BV605 Multimer
CCR7
95.69%
T TIME TIET
CD45RO
95.88%
JA TIET
PD-1+ Cells
Phenotype of MHC Multimer-Positive Cells
Effector Memory Phenotype
Tam
T₁
87.7%
PD-1
PD-1+ CD8 T cells
99.6%
CD8
C4D1
4.7%
193.89%
TE PYTING TING T
CPI, checkpoint inhibitor; PR, partial response; PD, progressive disease; SD, stable disease.
1. Sahin U, et al. Nature 2017; 547:222-226; BNT121 was a precursor to BNT122 and the iNeST collaboration with Genentech.
2. Lopez J, et al. AACR Annual Meeting 2020; Oral presentation CT301; 3. Braiteh F, et al. AACR Annual Meeting 2020; Poster presentation CT169; 4. Collaboration with Genentech.
Frequency of TCRs (log,0)
in Baseline Tumor
BNT122 induces CD8+ T cell
Infiltrates in tumors
-1.8-
-2-
-2.2
-24-
-2.6
-3.2
-34-
-3.6-
-3.8-
XODOO C
O
O
NUM
Content OOO O
mRNA cancer vaccines
0
RO7198457-specific TCRs
Other TCRs
RO7198457-specific
TCRs are present only
in post-treatment tumor.
●00000 COD
-36-34-32 -3 -28-26-24-22 -2 -18-16-4
Frequency of TCRs (log 10) in
Post-Treatment Tumor
BIONTECH
98View entire presentation