Kymera IPO Presentation Deck
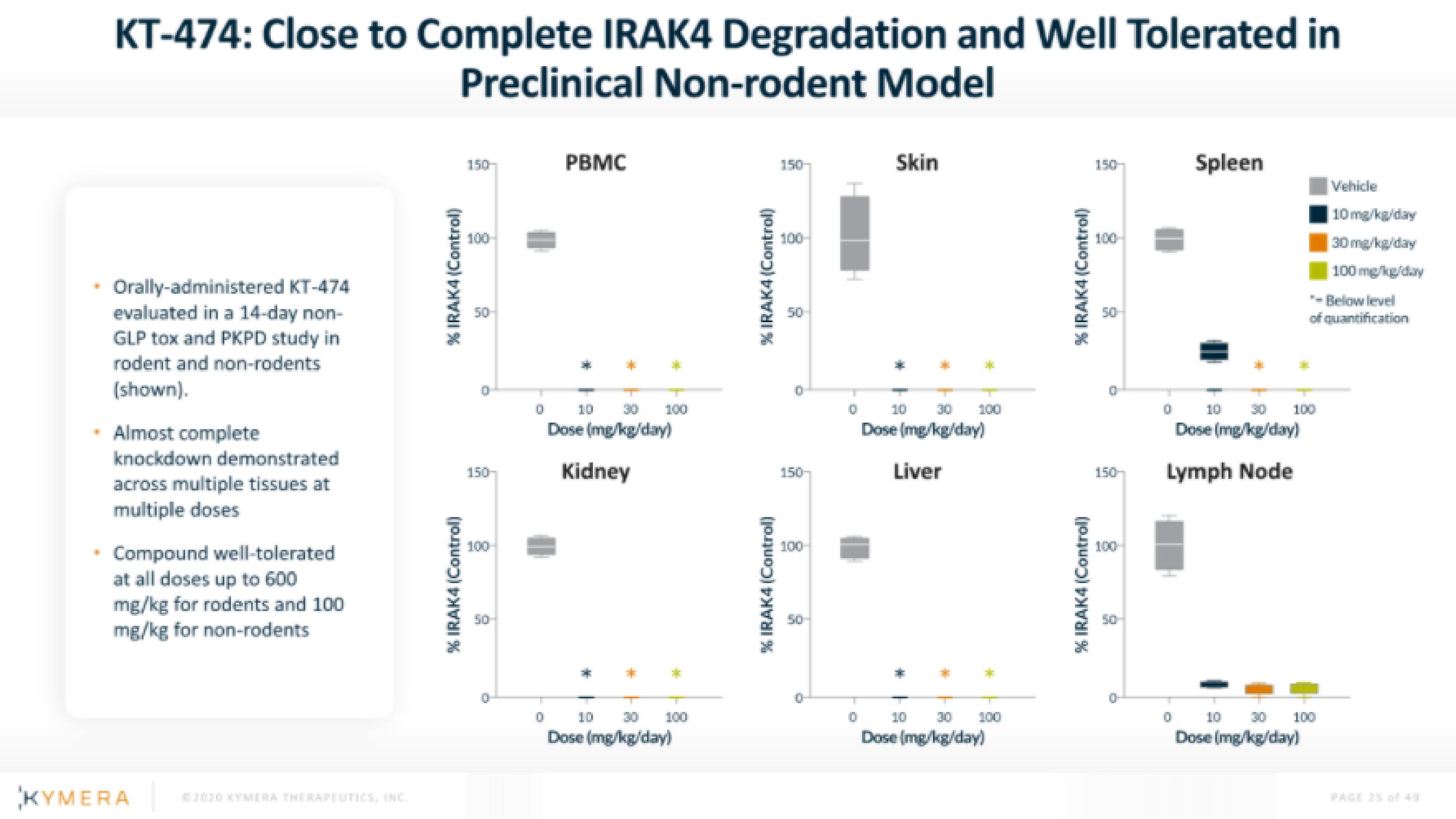
KT-474: Close to Complete IRAK4 Degradation and Well Tolerated in
Preclinical Non-rodent Model
• Orally-administered KT-474
evaluated in a 14-day non-
GLP tox and PKPD study in
rodent and non-rodents
(shown).
• Almost complete
knockdown demonstrated
across multiple tissues at
multiple doses
Compound well-tolerated
at all doses up to 600
mg/kg for rodents and 100
mg/kg for non-rodents
KYMERA
% IRAK4 (Control)
% IRAK4 (Control)
150-
50
150-
100
0
0
PBMC
10
30
Dose (mg/kg/day)
Kidney
Dose (mg/kg/day)
% IRAK4 (Control)
% IRAK4 (Control)
150-
150
100
10
Skin
Dose (mg/kg/day)
Liver
#
100
Dose (mg/kg/day)
% IRAK4 (Control)
% IRAK4 (Control)
150-
50
10
150-
2
Spleen
Dose (mg/kg/day)
Lymph Node
0
10
Dose (mg/kg/day)
Vehicle
10mg/kg/day
30 mg/kg/day
100 mg/kg/day
*- Below level
of quantificationView entire presentation