Corporate Presentation
Exploring the Broad Potential of Imetelstat
and Telomerase Inhibition
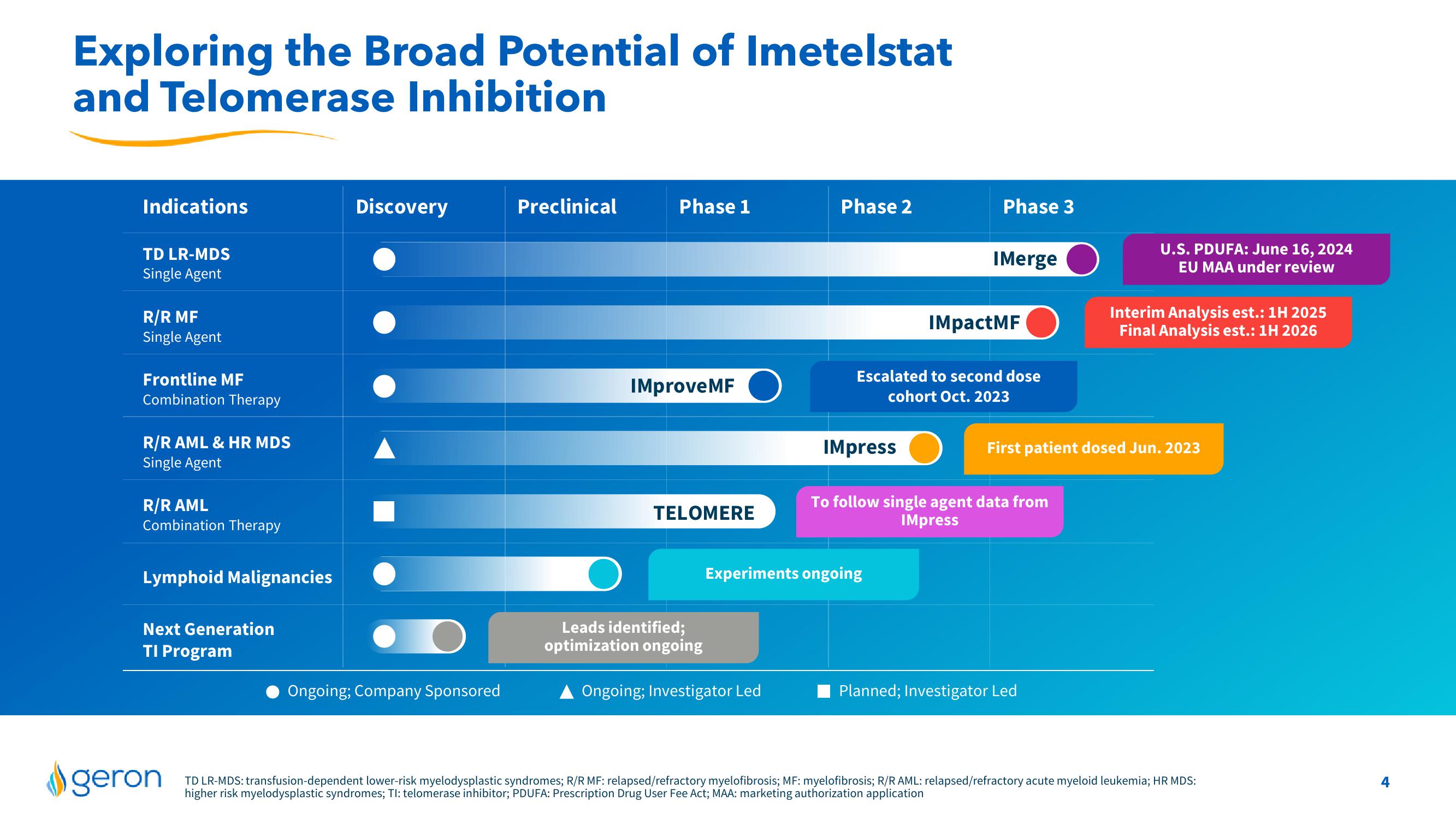
Indications
TD LR-MDS
Single Agent
R/R MF
Single Agent
Frontline MF
Combination Therapy
R/R AML & HR MDS
Single Agent
R/R AML
Combination Therapy
Lymphoid Malignancies
Next Generation
TI Program
geron
Discovery
Ongoing; Company Sponsored
Preclinical
Phase 1
IMproveMF
TELOMERE
Leads identified;
optimization ongoing
Phase 2
Ongoing; Investigator Led
IMpress
Phase 3
Experiments ongoing
IMerge
Escalated to second dose
cohort Oct. 2023
IMpactMF
To follow single agent data from
Impress
U.S. PDUFA: June 16, 2024
EU MAA under review
First patient dosed Jun. 2023
Planned; Investigator Led
Interim Analysis est.: 1H 2025
Final Analysis est.: 1H 2026
TD LR-MDS: transfusion-dependent lower-risk myelodysplastic syndromes; R/R MF: relapsed/refractory myelofibrosis; MF: myelofibrosis; R/R AML: relapsed/refractory acute myeloid leukemia; HR MDS:
higher risk myelodysplastic syndromes; Tl: telomerase inhibitor; PDUFA: Prescription Drug User Fee Act; MAA: marketing authorization applicationView entire presentation