Ocuphire Pharma Investor Presentation Deck
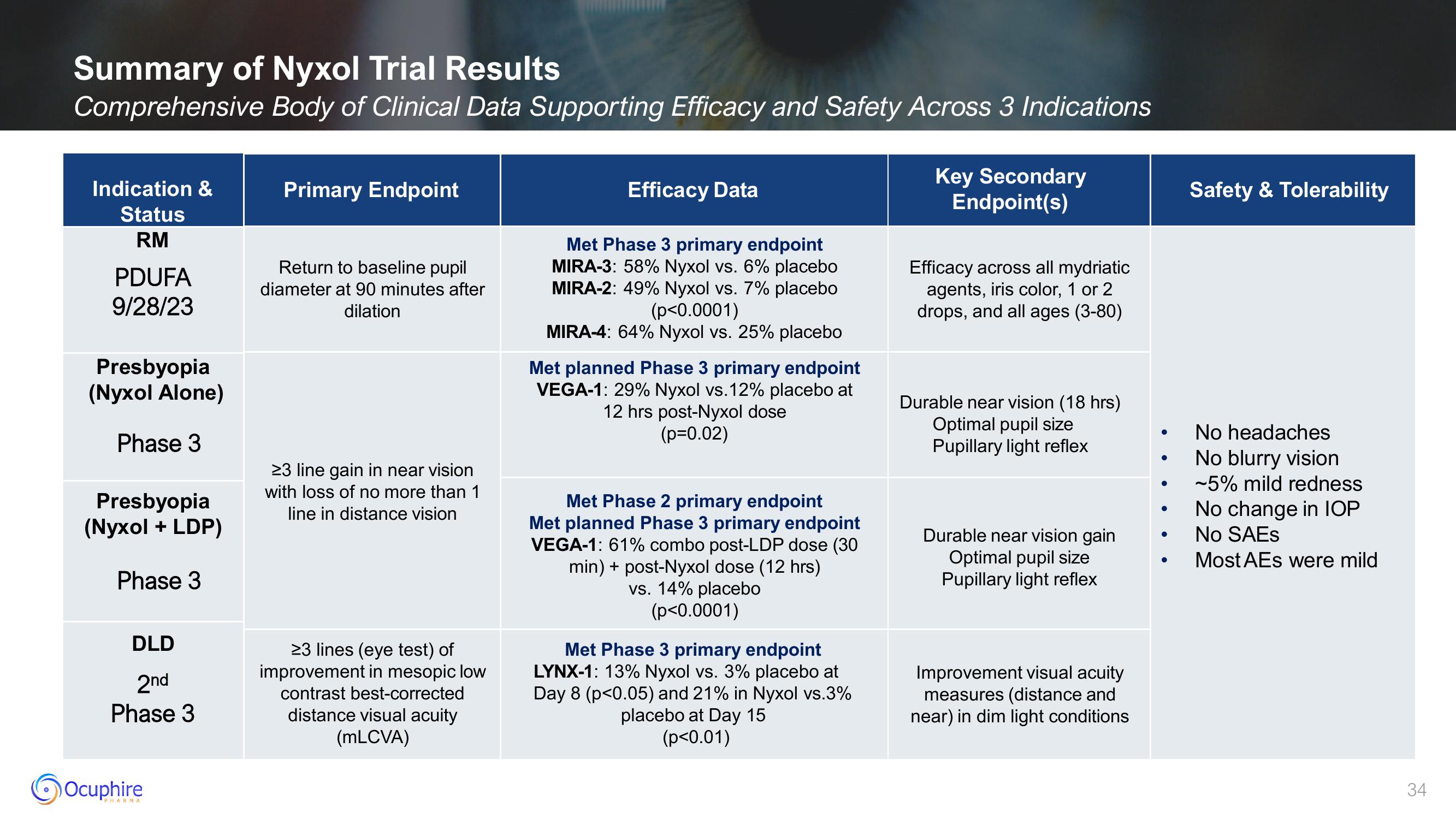
Summary of Nyxol Trial Results
Comprehensive Body of Clinical Data Supporting Efficacy and Safety Across 3 Indications
Indication &
Status
RM
PDUFA
9/28/23
Presbyopia
(Nyxol Alone)
Phase 3
Presbyopia
(Nyxol + LDP)
Phase 3
DLD
2nd
Phase 3
Ocuphire
Primary Endpoint
Return to baseline pupil
diameter at 90 minutes after
dilation
≥3 line gain in near vision
with loss of no more than 1
line in distance vision
23 lines (eye test) of
improvement in mesopic low
contrast best-corrected
distance visual acuity
(mLCVA)
Efficacy Data
Met Phase 3 primary endpoint
MIRA-3: 58% Nyxol vs. 6% placebo
MIRA-2: 49% Nyxol vs. 7% placebo
(p<0.0001)
MIRA-4: 64% Nyxol vs. 25% placebo
Met planned Phase 3 primary endpoint
VEGA-1: 29% Nyxol vs. 12% placebo at
12 hrs post-Nyxol dose
(p=0.02)
Met Phase 2 primary endpoint
Met planned Phase 3 primary endpoint
VEGA-1: 61% combo post-LDP dose (30
min) + post-Nyxol dose (12 hrs)
vs. 14% placebo
(p<0.0001)
Met Phase 3 primary endpoint
LYNX-1: 13% Nyxol vs. 3% placebo at
Day 8 (p<0.05) and 21% in Nyxol vs.3%
placebo at Day 15
(p<0.01)
Key Secondary
Endpoint(s)
Efficacy across all mydriatic
agents, iris color, 1 or 2
drops, and all ages (3-80)
Durable near vision (18 hrs)
Optimal pupil size
Pupillary light reflex
Durable near vision gain
Optimal pupil size
Pupillary light reflex
Improvement visual acuity
measures (distance and
near) in dim light conditions
Safety & Tolerability
No headaches
No blurry vision
~5% mild redness
No change in IOP
No SAEs
Most AEs were mild
34View entire presentation