Ocuphire Pharma Investor Updates
P
LO
5
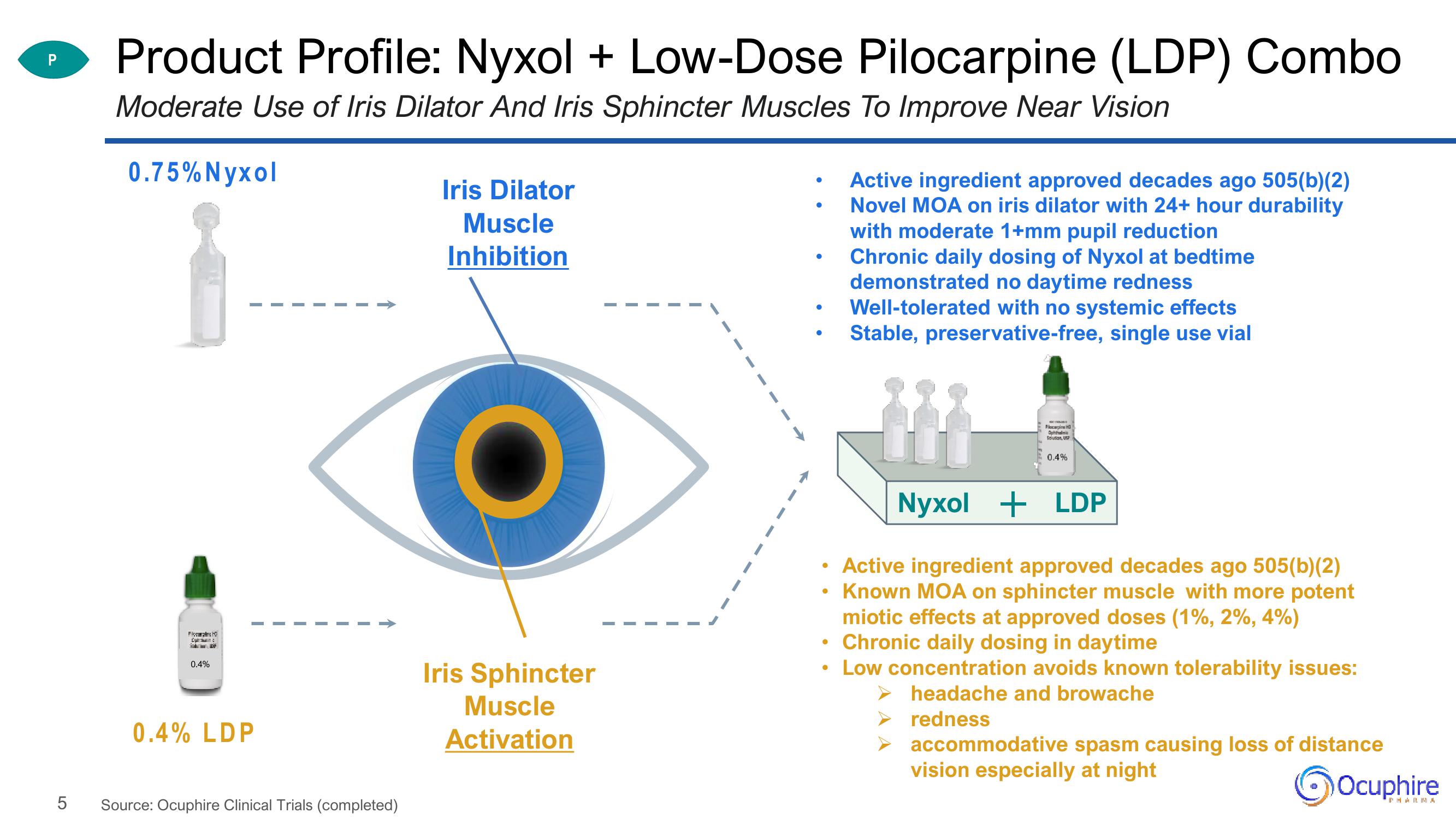
Product Profile: Nyxol + Low-Dose Pilocarpine (LDP) Combo
Moderate Use of Iris Dilator And Iris Sphincter Muscles To Improve Near Vision
0.75% Nyxol
F
O
0.4%
0.4% LDP
Source: Ocuphire Clinical Trials (completed)
Iris Dilator
Muscle
Inhibition
Iris Sphincter
Muscle
Activation
●
●
●
●
●
Active ingredient approved decades ago 505(b)(2)
Novel MOA on iris dilator with 24+ hour durability
with moderate 1+mm pupil reduction
Chronic daily dosing of Nyxol at bedtime
demonstrated no daytime redness
Well-tolerated with no systemic effects
Stable, preservative-free, single use vial
Ⓡ
Pilocarpine
Opal
Solution, US
0.4%
• Active ingredient approved decades ago 505(b)(2)
Known MOA on sphincter muscle with more potent
miotic effects at approved doses (1%, 2%, 4%)
Chronic daily dosing in daytime
Low concentration avoids known tolerability issues:
headache and browache
➤
redness
Nyxol + LDP
accommodative spasm causing loss of distance
vision especially at night
Ocuphire
PHARMAView entire presentation