Ocuphire Pharma Results Presentation Deck
RM
18
• There were no deaths, serious AEs, or withdrawals due to AEs
Only AEs, occurring in ≥ 5% of subjects treated with Nyxol, were instillation site
discomfort (38% Nyxol vs. 9% placebo) and conjunctival hyperemia (13% Nyxol vs. 0%
placebo)
●
Secondary Endpoint: Safety Findings
Nyxol was Well Tolerated with a Favorable Safety Profile
●
94% of the AEs in the Nyxol group were mild
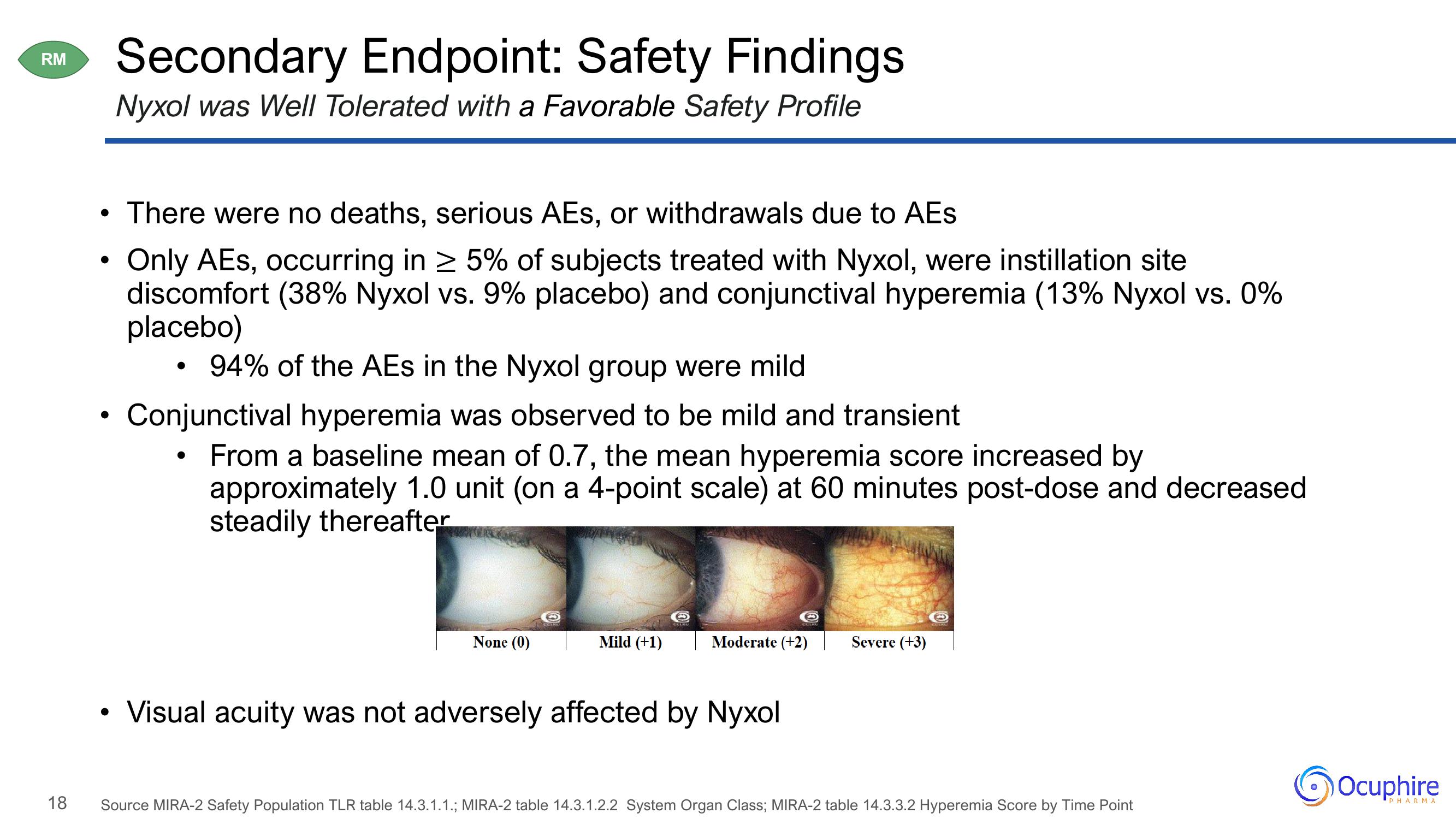
Conjunctival hyperemia was observed to be mild and transient
From a baseline mean of 0.7, the mean hyperemia score increased by
approximately 1.0 unit (on a 4-point scale) at 60 minutes post-dose and decreased
steadily thereafter
●
None (0)
Mild (+1)
6
Moderate (+2)
Visual acuity was not adversely affected by Nyxol
Severe (+3)
Source MIRA-2 Safety Population TLR table 14.3.1.1.; MIRA-2 table 14.3.1.2.2 System Organ Class; MIRA-2 table 14.3.3.2 Hyperemia Score by Time Point
Ocuphire
PHARMAView entire presentation