Kymera Investor Presentation Deck
0
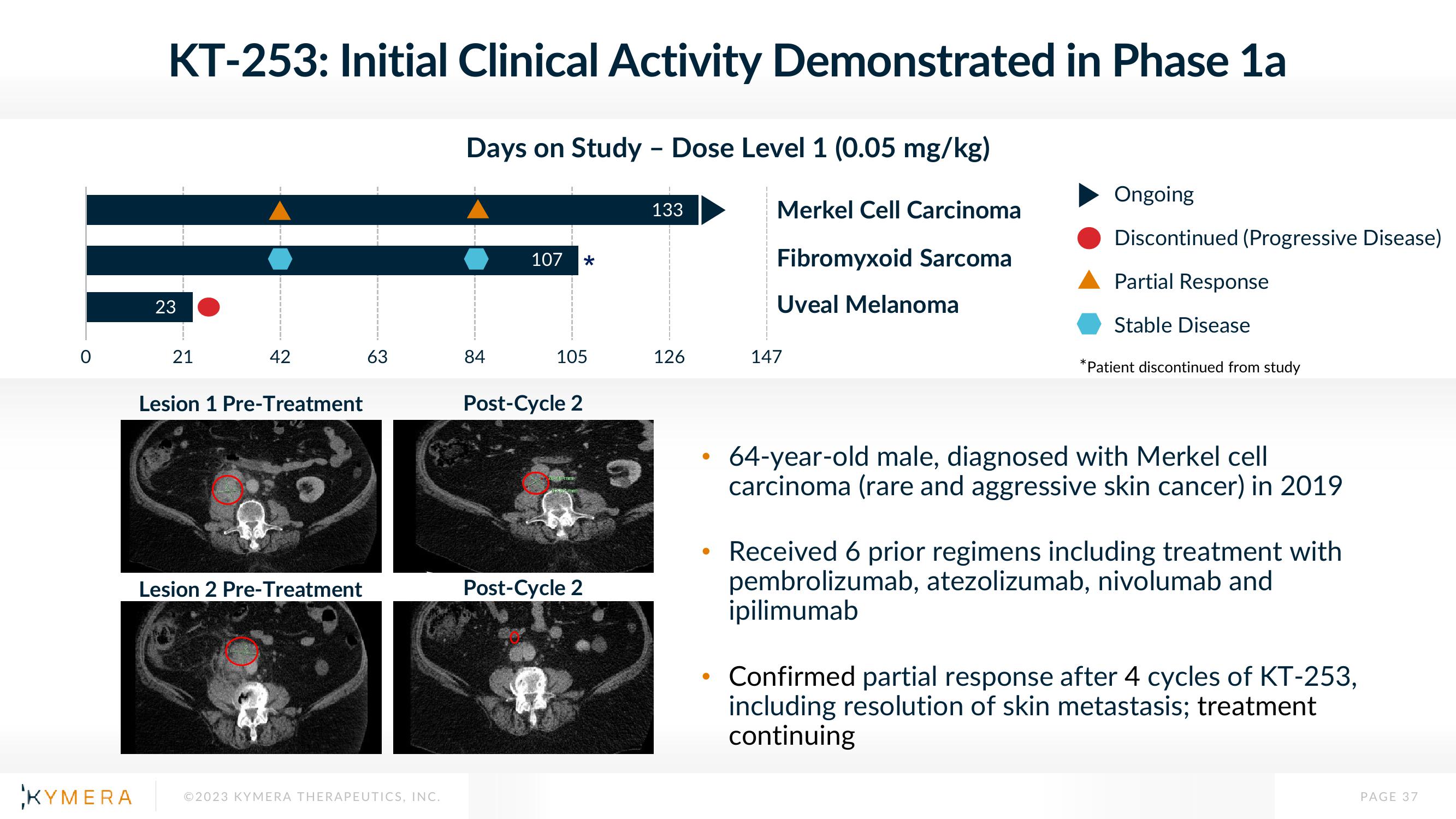
KT-253: Initial Clinical Activity Demonstrated in Phase 1a
23
21
42
Lesion 1 Pre-Treatment
Lesion 2 Pre-Treatment
63
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
Days on Study - Dose Level 1 (0.05 mg/kg)
84
107 *
105
Post-Cycle 2
Post-Cycle 2
133
126
Merkel Cell Carcinoma
Fibromyxoid Sarcoma
Uveal Melanoma
147
Ongoing
Discontinued (Progressive Disease)
Partial Response
Stable Disease
*Patient discontinued from study
64-year-old male, diagnosed with Merkel cell
carcinoma (rare and aggressive skin cancer) in 2019
Received 6 prior regimens including treatment with
pembrolizumab, atezolizumab, nivolumab and
ipilimumab
Confirmed partial response after 4 cycles of KT-253,
including resolution of skin metastasis; treatment
continuing
PAGE 37View entire presentation