Immix Biopharma Investor Presentation Deck
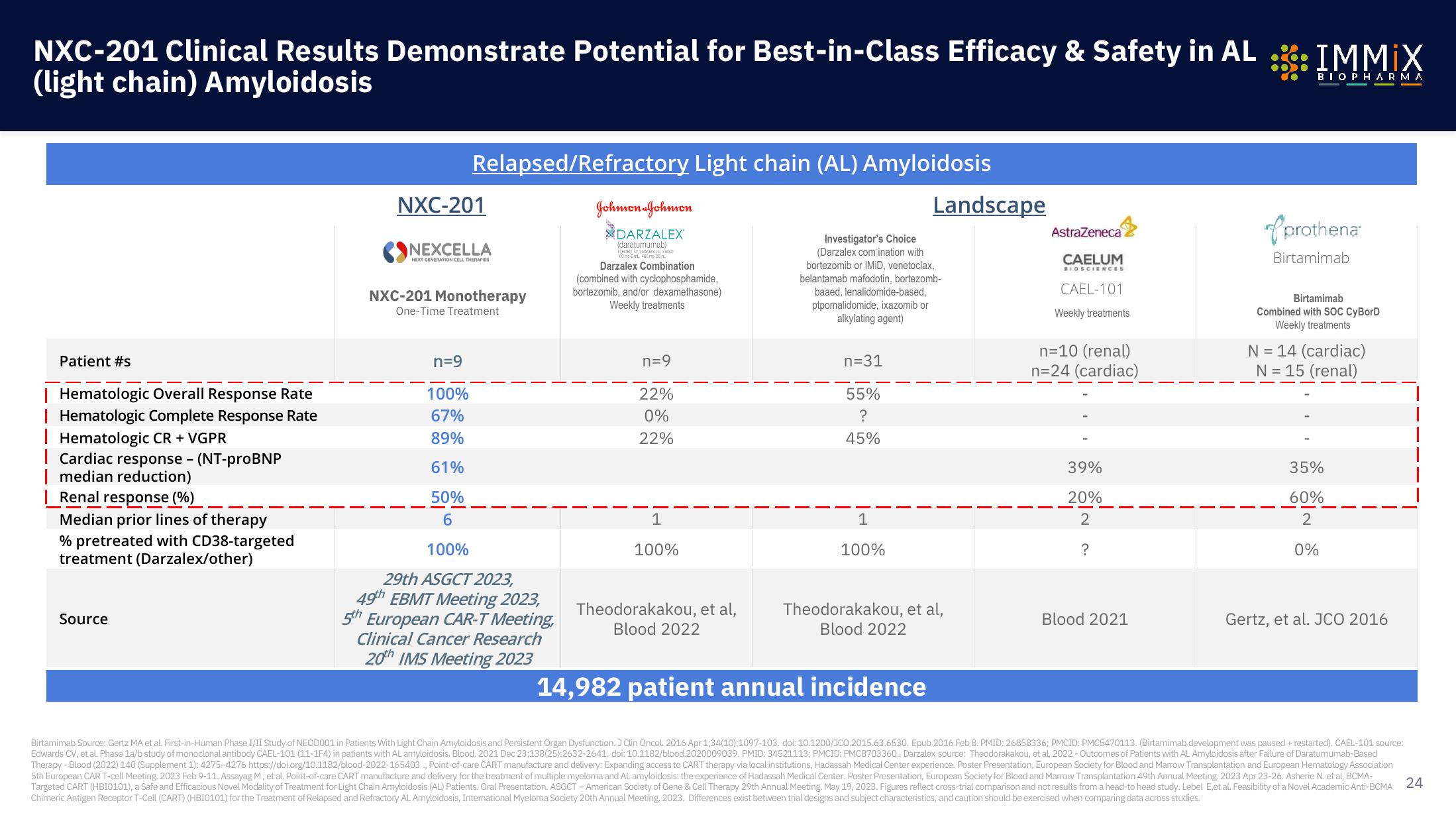
NXC-201 Clinical Results Demonstrate Potential for Best-in-Class Efficacy & Safety in AL
(light chain) Amyloidosis
Patient #s
Hematologic Overall Response Rate
| Hematologic Complete Response Rate
Hematologic CR + VGPR
I Cardiac response - (NT-proBNP
median reduction)
Renal response (%)
Median prior lines of therapy
% pretreated with CD38-targeted
treatment (Darzalex/other)
Relapsed/Refractory Light chain (AL) Amyloidosis
Source
NXC-201
NEXCELLA
NEXT GENERATION CELL THERAPIES
NXC-201 Monotherapy
One-Time Treatment
n=9
100%
67%
89%
61%
50%
6
100%
29th ASGCT 2023,
49th EBMT Meeting 2023,
5th European CAR-T Meeting,
Clinical Cancer Research
20th IMS Meeting 2023
Johnson & Johnson
DARZALEX
(daratumumab)
i
Darzalex Combination
(combined with cyclophosphamide,
bortezomib, and/or dexamethasone)
Weekly treatments
n=9
22%
0%
22%
1
100%
Investigator's Choice
(Darzalex comination with
bortezomib or IMiD, venetoclax,
belantamab mafodotin, bortezomb-
baaed, lenalidomide-based,
ptpomalidomide, ixazomib or
alkylating agent)
n=31
55%
?
45%
Landscape
1
100%
AstraZeneca
CAELUM
BIOSCIENCES
CAEL-101
Weekly treatments
n=10 (renal)
n=24 (cardiac)
39%
20%
2
?
●●●
S
Blood 2021
IMMİX
BIOPHARMA
prothena
Birtamimab
Birtamimab
Combined with SOC CyBorD
Weekly treatments
N = 14 (cardiac)
N = 15 (renal)
Theodorakakou, et al,
Blood 2022
Theodorakakou, et al,
Blood 2022
14,982 patient annual incidence
Birtamimab Source: Gertz MA et al. First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction. J Clin Oncol. 2016 Apr 1;34(10):1097-103. doi: 10.1200/JCO.2015.63.6530. Epub 2016 Feb 8. PMID: 26858336; PMCID: PMC5470113. (Birtamimab development was paused + restarted). CAEL-101 source:
Edwards CV, et al. Phase 1a/b study of monoclonal antibody CAEL-101 (11-1F4) in patients with AL amyloidosis. Blood. 2021 Dec 23;138(25):2632-2641. doi: 10.1182/blood.2020009039. PMID: 34521113; PMCID: PMC8703360.. Darzalex source: Theodorakakou, et al, 2022 - Outcomes of Patients with AL Amyloidosis after Failure of Daratumumab-Based
Therapy-Blood (2022) 140 (Supplement 1): 4275-4276 https://doi.org/10.1182/blood-2022-165403, Point-of-care CART manufacture and delivery: Expanding access to CART therapy via local institutions, Hadassah Medical Center experience. Poster Presentation, European Society for Blood and Marrow Transplantation and European Hematology Association
5th European CAR T-cell Meeting. 2023 Feb 9-11. Assayag M, et al. Point-of-care CART manufacture and delivery for the treatment of multiple myeloma and AL amyloidosis: the experience of Hadassah Medical Center. Poster Presentation, European Society for Blood and Marrow Transplantation 49th Annual Meeting. 2023 Apr 23-26. Asherie N. et al, BCMA-
Targeted CART (HBI0101), a Safe and Efficacious Novel Modality of Treatment for Light Chain Amyloidosis (AL) Patients. Oral Presentation. ASGCT-American Society of Gene & Cell Therapy 29th Annual Meeting. May 19, 2023. Figures reflect cross-trial comparison and not results from a head-to head study. Lebel E,et al. Feasibility of a Novel Academic Anti-BCMA 24
Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory AL Amyloidosis, International Myeloma Society 20th Annual Meeting, 2023. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies.
35%
60%
2
0%
Gertz, et al. JCO 2016View entire presentation