Strategic Transformation 2023
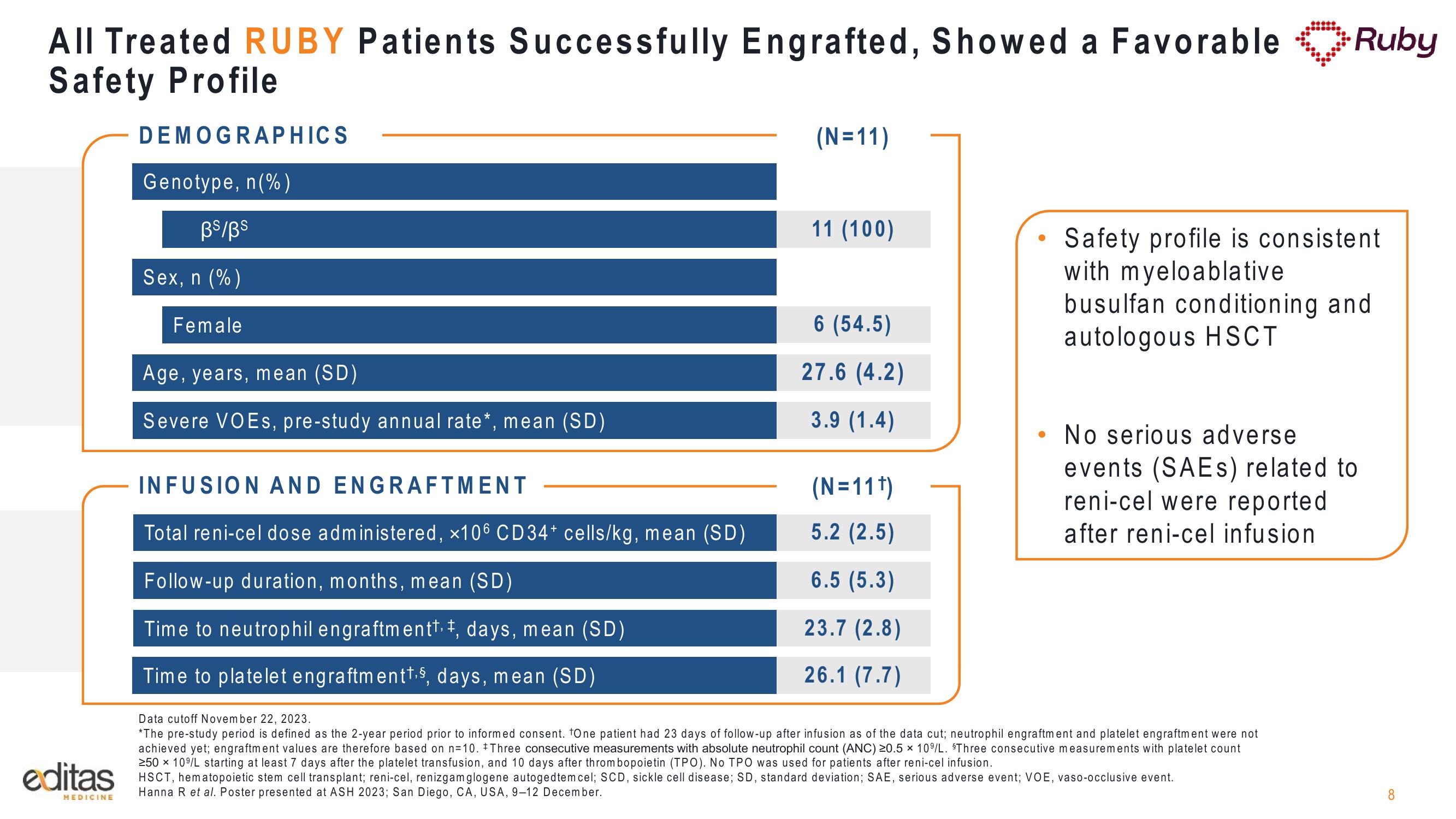
All Treated RUBY Patients Successfully Engrafted, Showed a Favorable Ruby
Safety Profile
editas
MEDICINE
DEMOGRAPHICS
Genotype, n (%)
BS/Bs
Sex, n (%)
Female
Age, years, mean (SD)
Severe VOEs, pre-study annual rate*, mean (SD)
INFUSION AND ENGRAFTMENT
Total reni-cel dose administered, x106 CD34+ cells/kg, mean (SD)
Follow-up duration, months, mean (SD)
Time to neutrophil engraftmentt. ‡, days, mean (SD)
Time to platelet engraftment¹.⁹, days, mean (SD)
(N=11)
11 (100)
6 (54.5)
27.6 (4.2)
3.9 (1.4)
(N=11¹)
5.2 (2.5)
6.5 (5.3)
23.7 (2.8)
26.1 (7.7)
Safety profile is consistent.
with myeloablative
busulfan conditioning and
autologous HSCT
No serious adverse
events (SAES) related to
reni-cel were reported
after reni-cel infusion.
Data cutoff November 22, 2023.
*The pre-study period is defined as the 2-year period prior to informed consent. One patient had 23 days of follow-up after infusion as of the data cut; neutrophil engraftment and platelet engraftment were not
achieved yet; engraftment values are therefore based on n=10. *Three consecutive measurements with absolute neutrophil count (ANC) 20.5 × 10%/L. $Three consecutive measurements with platelet count
250 × 10%/L starting at least 7 days after the platelet transfusion, and 10 days after thrombopoietin (TPO). No TPO was used for patients after reni-cel infusion.
HSCT, hematopoietic stem cell transplant; reni-cel, renizgam glogene autogedtem cel; SCD, sickle cell disease; SD, standard deviation; SAE, serious adverse event; VOE, vaso-occlusive event.
Hanna R et al. Poster presented at ASH 2023; San Diego, CA, USA, 9-12 December.
8View entire presentation