Imara M&A
●
●
●
Significant Need Remains for More Treatment Options for CML
Challenges with Current Standard of Care
Approximately 1 in 5 patients switch therapy within the first year
and ~40% of patients switch in the first 5 years (1L & 2L)
Growing 3L+ patient population (>25% of CP-CML) with limited
treatment options
Except for asciminib, the approved TKIs have poor kinase
selectivity resulting in tolerability issues that impact efficacy
Comorbidities, restrictions with concomitant medications,
and specific administration requirements impede long-term
patient adherence
• Fewer than 10% of patients successfully achieve sustained
treatment-free remission (TFR)
Majority of HCPs (77%) indicated need for
more effective, safe, and tolerable agents in CML
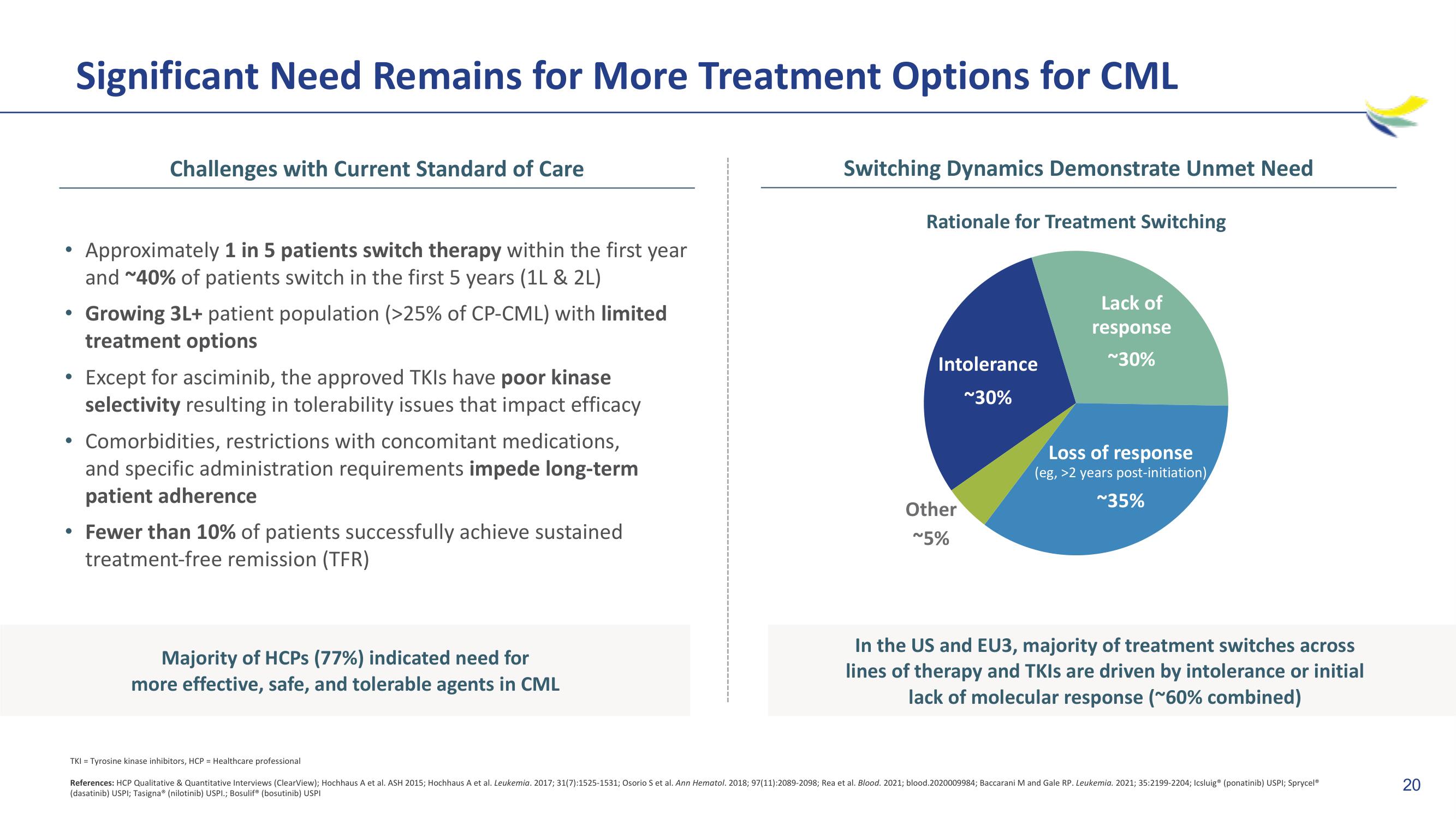
Switching Dynamics Demonstrate Unmet Need
Rationale for Treatment Switching
Intolerance
~30%
Other
~5%
Lack of
response
~30%
Loss of response
(eg, >2 years post-initiation)
~35%
In the US and EU3, majority of treatment switches across
lines of therapy and TKIs are driven by intolerance or initial
lack of molecular response (~60% combined)
TKI = Tyrosine kinase inhibitors, HCP= Healthcare professional
References: HCP Qualitative & Quantitative Interviews (ClearView); Hochhaus A et al. ASH 2015; Hochhaus A et al. Leukemia. 2017; 31(7):1525-1531; Osorio S et al. Ann Hematol. 2018; 97(11):2089-2098; Rea et al. Blood. 2021; blood.2020009984; Baccarani M and Gale RP. Leukemia. 2021; 35:2199-2204; Icsluig® (ponatinib) USPI; Sprycel®
(dasatinib) USPI; Tasigna® (nilotinib) USPI.; Bosulife (bosutinib) USPI
20View entire presentation