Ocuphire Pharma Results Presentation Deck
Percent of Subjects (%)
Percent of Subjects With Binocular Worsening in DRSS of ≥ 3-Step
Selected Primary Registration Endpoint for Phase 3, To Be Formally Confirmed at EOP2 FDA Meeting
20%
15%
10%
5%
0%
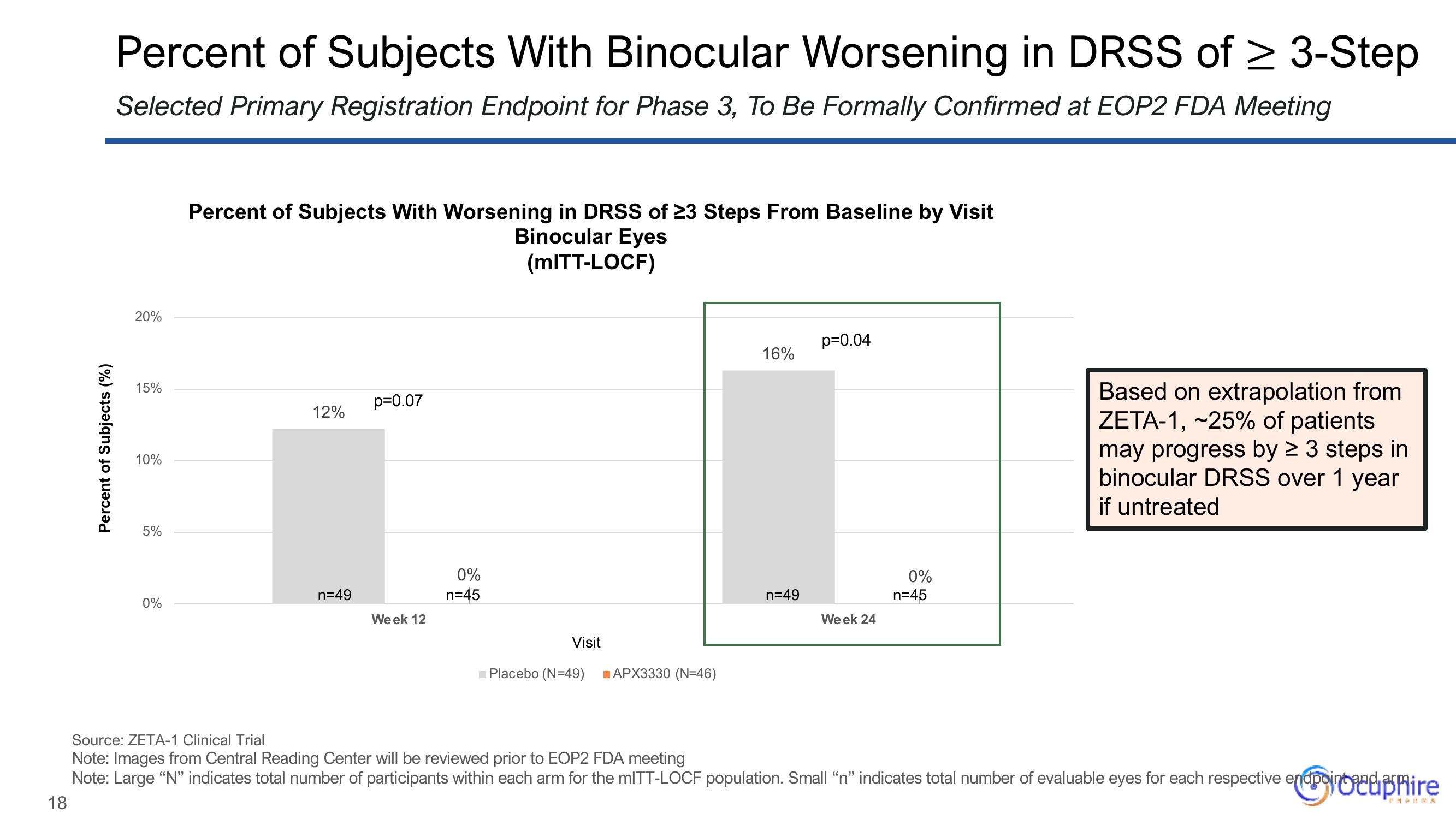
Percent of Subjects With Worsening in DRSS of 23 Steps From Baseline by Visit
Binocular Eyes
(MITT-LOCF)
12%
n=49
p=0.07
Week 12
0%
n=45
Visit
Placebo (N=49) APX3330 (N=46)
16%
n=49
p=0.04
Week 24
0%
n=45
Based on extrapolation from
ZETA-1, -25% of patients
may progress by ≥ 3 steps in
binocular DRSS over 1 year
if untreated
Source: ZETA-1 Clinical Trial
Note: Images from Central Reading Center will be reviewed prior to EOP2 FDA meeting
Note: Large "N" indicates total number of participants within each arm for the mITT-LOCF population. Small "n" indicates total number of evaluable eyes for each respective endpoint and arm:
Predprire
18View entire presentation