Certara Investor Presentation Deck
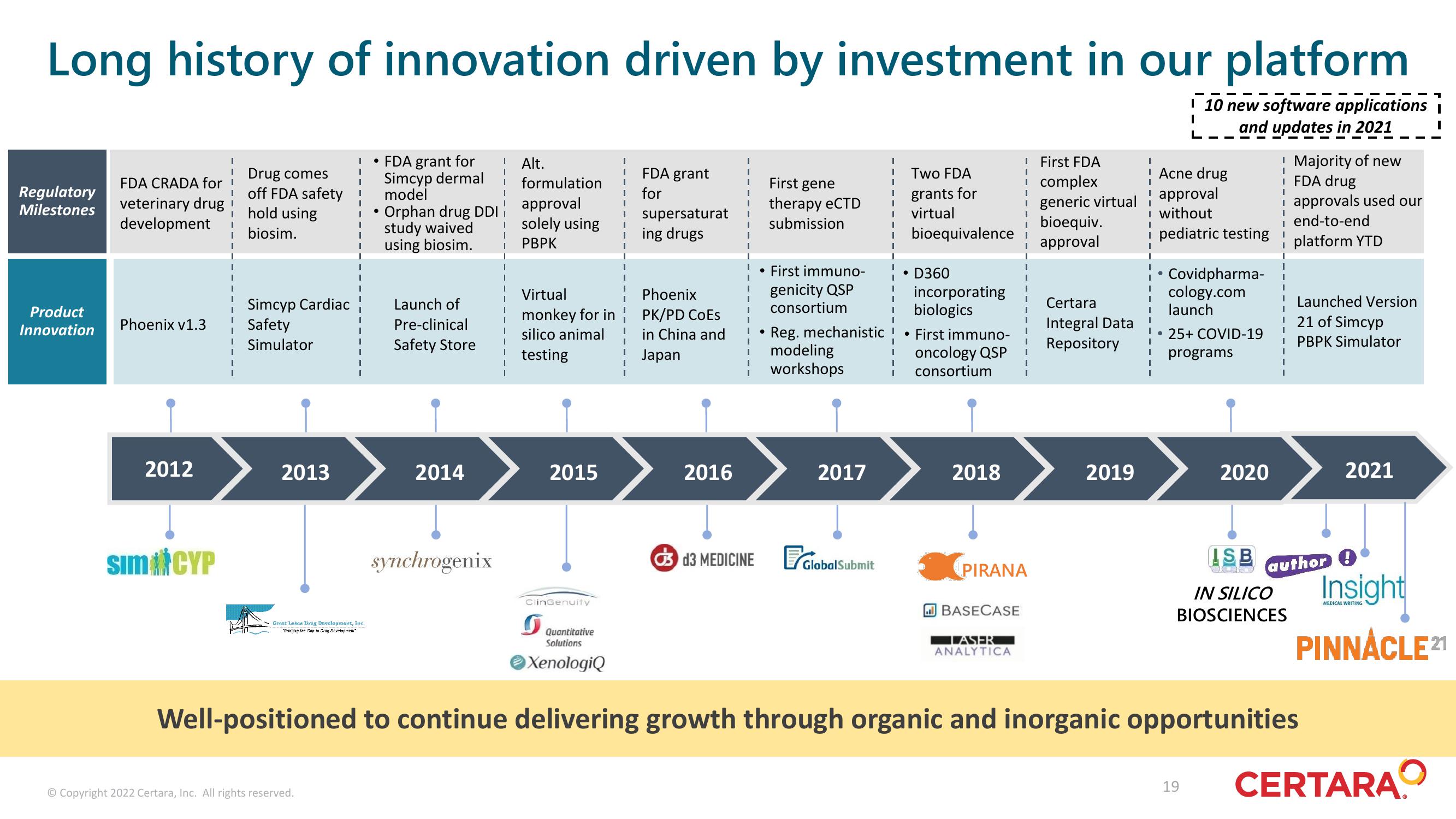
Long history of innovation driven by investment in our platform
I 10 new software applications i
| and updates in 2021
1
Regulatory
Milestones
Product
Innovation
FDA CRADA for
veterinary drug
development
Phoenix v1.3
2012
Sim CYP
Drug comes
off FDA safety
hold using
biosim.
Simcyp Cardiac
Safety
Simulator
2013
I
I
© Copyright 2022 Certara, Inc. All rights reserved.
I
I
Great Lakes Brug Development, Inc.
"Bridging the Gap in Drug Development"
●
●
FDA grant for
Simcyp dermal
model
Orphan drug DDI
study waived
using biosim.
Launch of
Pre-clinical
Safety Store
2014
synchrogenix
formulation
approval
isolely using
PBPK
I
Alt.
I
|
Virtual
monkey for in
silico animal
testing
2015
ClinGenuity
G
Quantitative
Solutions
XenologiQ
I
I
FDA grant
for
supersaturat
ing drugs
Phoenix
PK/PD CoEs
in China and
Japan
2016
I
I
| First gene
I
I
I
I
therapy eCTD
submission
• First immuno-
genicity QSP
consortium
●
2017
I
d3 MEDICINE Global Submit
GlobalSubmit
I
Two FDA
grants for
virtual
bioequivalence i
• D360
I
Reg. mechanistic ! • First immuno-
modeling
oncology QSP
workshops
incorporating
biologics
I
I consortium
2018
! First FDA
i complex
i generic virtual
i bioequiv.
approval
BASECASE
LASER
ANALYTICA
I
PIRANA
Certara
Integral Data
Repository
2019
I
! Acne drug
I
approval
without
i pediatric testing
I
I
Covidpharma- I
cology.com
launch
! Launched Version
25+ COVID-19
programs
2020
! Majority of new
i FDA drug
I
i approvals used our
I
end-to-end
platform YTD
ISB
200
19
i
I
I
I
I
IN SILICO
BIOSCIENCES
21 of Simcyp
PBPK Simulator
author
Well-positioned to continue delivering growth through organic and inorganic opportunities
2021
MEDICAL WRITING
Insight
PINNACLE²¹
CERTARAView entire presentation