BioAtla Investor Presentation Deck
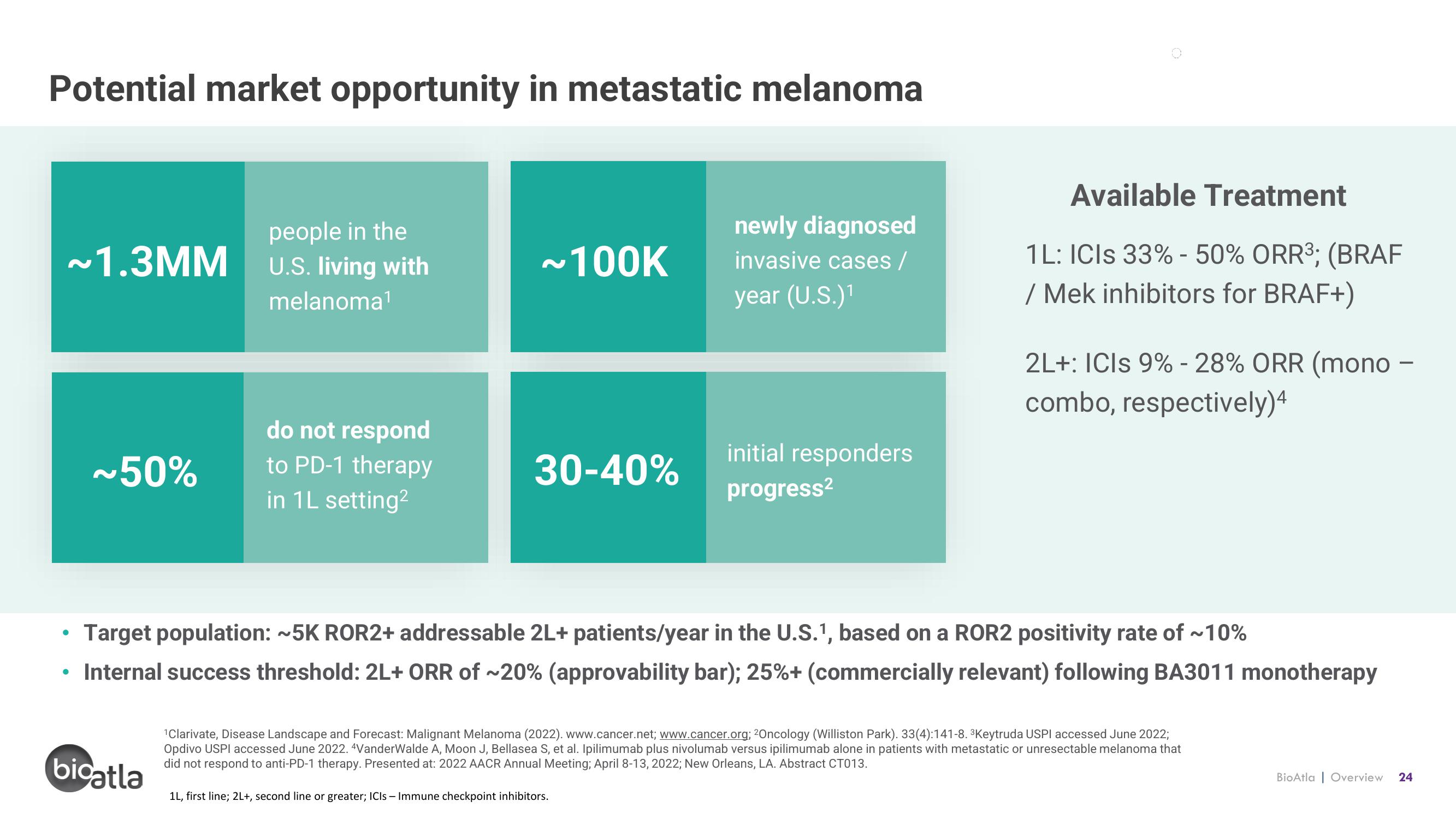
Potential market opportunity in metastatic melanoma
~1.3MM
●
●
~50%
people in the
U.S. living with
melanoma¹
bicatla
do not respond
to PD-1 therapy
in 1L setting²
~100K
30-40%
newly diagnosed
invasive cases /
year (U.S.)¹
initial responders
progress²
1L, first line; 2L+, second line or greater; ICIS - Immune checkpoint inhibitors.
Available Treatment
1L: ICIs 33% - 50% ORR³; (BRAF
/ Mek inhibitors for BRAF+)
Target population: ~5K ROR2+ addressable 2L+ patients/year in the U.S.1, based on a ROR2 positivity rate of ~10%
Internal success threshold: 2L+ ORR of ~20% (approvability bar); 25%+ (commercially relevant) following BA3011 monotherapy
2L+: ICIS 9% -28% ORR (mono -
combo, respectively)4
¹Clarivate, Disease Landscape and Forecast: Malignant Melanoma (2022). www.cancer.net; www.cancer.org; 2Oncology (Williston Park). 33(4):141-8. ³Keytruda USPI accessed June 2022;
Opdivo USPI accessed June 2022. 4VanderWalde A, Moon J, Bellasea S, et al. Ipilimumab plus nivolumab versus ipilimumab alone in patients with metastatic or unresectable melanoma that
did not respond to anti-PD-1 therapy. Presented at: 2022 AACR Annual Meeting; April 8-13, 2022; New Orleans, LA. Abstract CT013.
BioAtla| Overview 24View entire presentation