AbCellera Investor Presentation Deck
FINANCIALS
CORPORATE OVERVIEW
COPYRIGHT © ABCELLERA
18
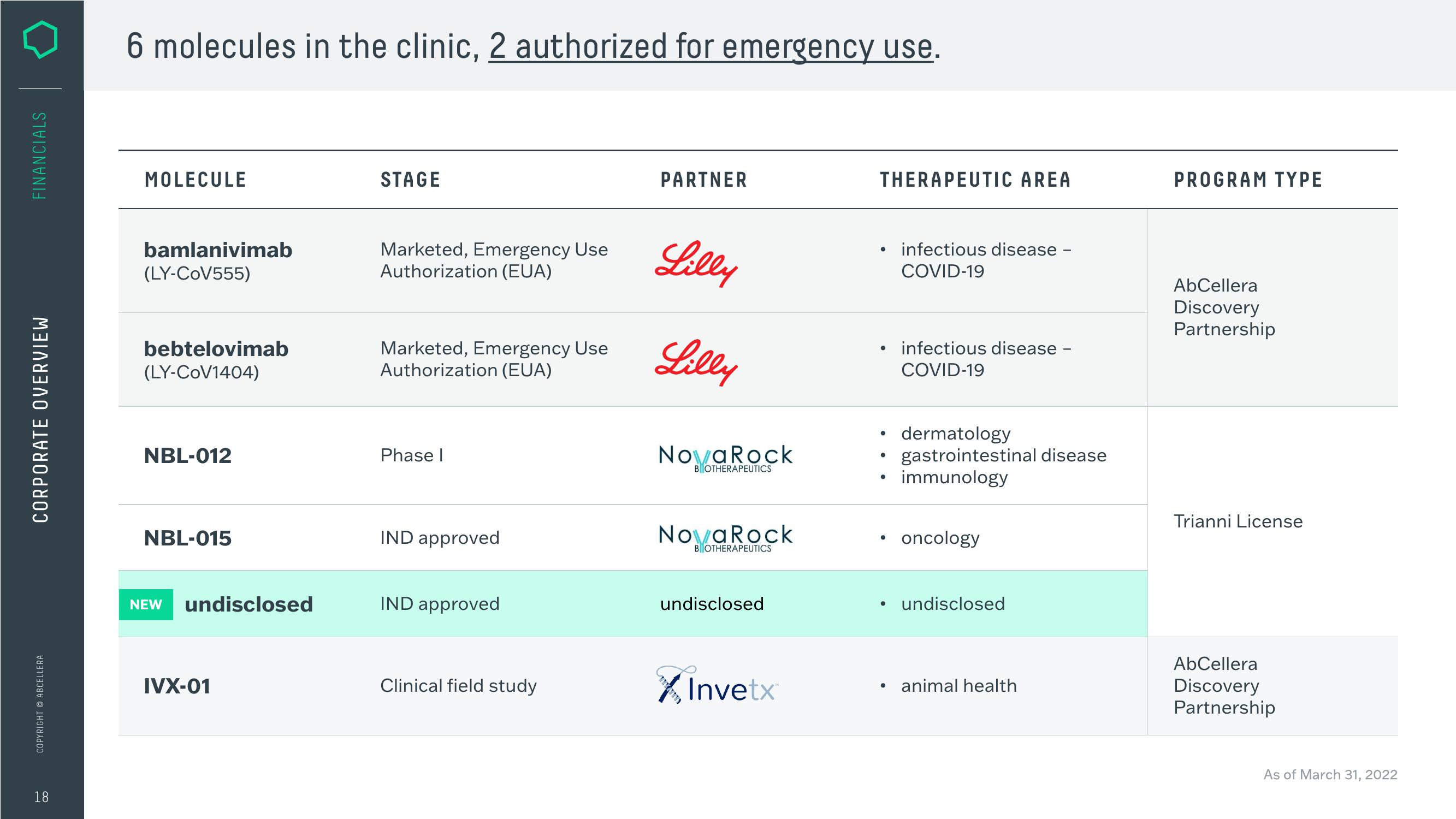
6 molecules in the clinic, 2 authorized for emergency use.
MOLECULE
bamlanivimab
(LY-CoV555)
bebtelovimab
(LY-CoV1404)
NBL-012
NBL-015
NEW undisclosed
IVX-01
STAGE
Marketed, Emergency Use
Authorization (EUA)
Marketed, Emergency Use
Authorization (EUA)
Phase I
IND approved
IND approved
Clinical field study
PARTNER
Lilly
Lilly
NovaRock
BOTHERAPEUTICS
NovaRock
BOTHERAPEUTICS
undisclosed
Invetx™
THERAPEUTIC AREA
●
●
●
●
●
●
infectious disease -
COVID-19
infectious disease -
COVID-19
dermatology
gastrointestinal disease
immunology
oncology
undisclosed
animal health
PROGRAM TYPE
AbCellera
Discovery
Partnership
Trianni License
AbCellera
Discovery
Partnership
As of March 31, 2022View entire presentation