Immix Biopharma Investor Presentation Deck
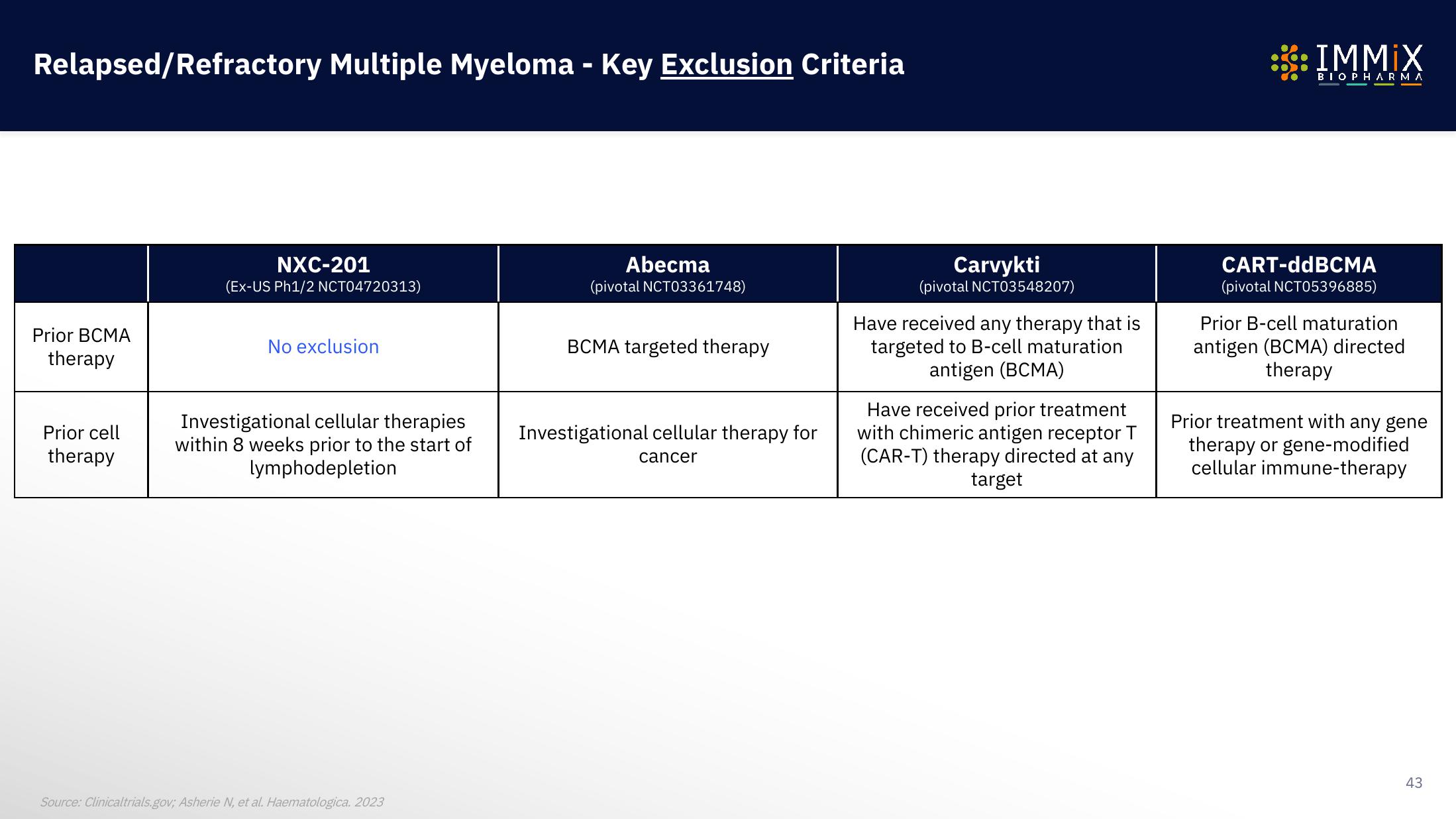
Relapsed/Refractory Multiple Myeloma - Key Exclusion Criteria
Prior BCMA
therapy
Prior cell
therapy
NXC-201
(Ex-US Ph1/2 NCT04720313)
No exclusion
Investigational cellular therapies
within 8 weeks prior to the start of
lymphodepletion
Source: Clinical trials.gov; Asherie N, et al. Haematologica. 2023
Abecma
(pivotal NCT03361748)
BCMA targeted therapy
Investigational cellular therapy for
cancer
Carvykti
(pivotal NCT03548207)
Have received any therapy that is
targeted to B-cell maturation
antigen (BCMA)
Have received prior treatment
with chimeric antigen receptor T
(CAR-T) therapy directed at any
target
●●●
S
IMMIX
BIOPHARMA
CART-ddBCMA
(pivotal NCT05396885)
Prior B-cell maturation
antigen (BCMA) directed
therapy
Prior treatment with any gene
therapy or gene-modified
cellular immune-therapy
43View entire presentation