BioAtla Investor Presentation Deck
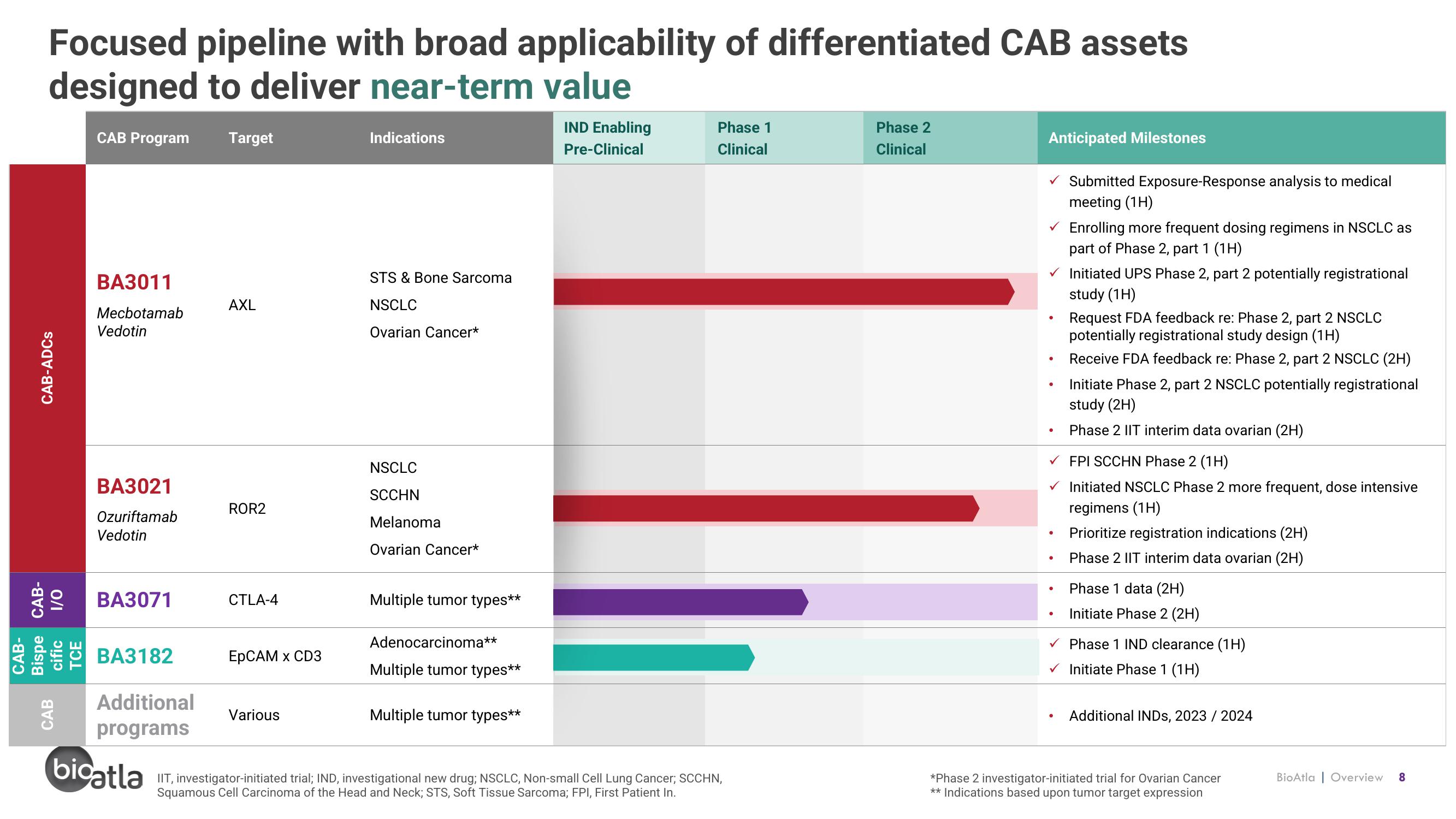
Focused pipeline with broad applicability of differentiated CAB assets
designed to deliver near-term value
CAB-ADCs
Bispe CAB-
CAB-
cific
TCE
O/I
CAB
CAB Program
BA3011
Mecbotamab
Vedotin
BA3021
Ozuriftamab
Vedotin
BA3071
BA3182
Additional
programs
bicatla
Target
AXL
ROR2
CTLA-4
EpCAM x CD3
Various
Indications
STS & Bone Sarcoma
NSCLC
Ovarian Cancer*
NSCLC
SCCHN
Melanoma
Ovarian Cancer*
Multiple tumor types**
Adenocarcinoma**
Multiple tumor types**
Multiple tumor types**
IND Enabling
Pre-Clinical
Phase 1
Clinical
IIT, investigator-initiated trial; IND, investigational new drug; NSCLC, Non-small Cell Lung Cancer; SCCHN,
Squamous Cell Carcinoma of the Head and Neck; STS, Soft Tissue Sarcoma; FPI, First Patient In.
Phase 2
Clinical
Anticipated Milestones
●
✓ Initiated UPS Phase 2, part 2 potentially registrational
study (1H)
●
●
Submitted Exposure-Response analysis to medical
meeting (1H)
Enrolling more frequent dosing regimens in NSCLC as
part of Phase 2, part 1 (1H)
●
Request FDA feedback re: Phase 2, part 2 NSCLC
potentially registrational study design (1H)
Receive FDA feedback re: Phase 2, part 2 NSCLC (2H)
Initiate Phase 2, part 2 NSCLC potentially registrational
study (2H)
Phase 2 IIT interim data ovarian (2H)
✓ FPI SCCHN Phase 2 (1H)
Initiated NSCLC Phase 2 more frequent, dose intensive
regimens (1H)
Prioritize registration indications (2H)
Phase 2 IIT interim data ovarian (2H)
Phase 1 data (2H)
Initiate Phase 2 (2H)
✓ Phase 1 IND clearance (1H)
✓ Initiate Phase 1 (1H)
Additional INDS, 2023 / 2024
*Phase 2 investigator-initiated trial for Ovarian Cancer
** Indications based upon tumor target expression
BioAtla| Overview 8View entire presentation