Calliditas Therapeutics IPO Presentation Deck
Commercialization strategy and market opportunity in the U.S.
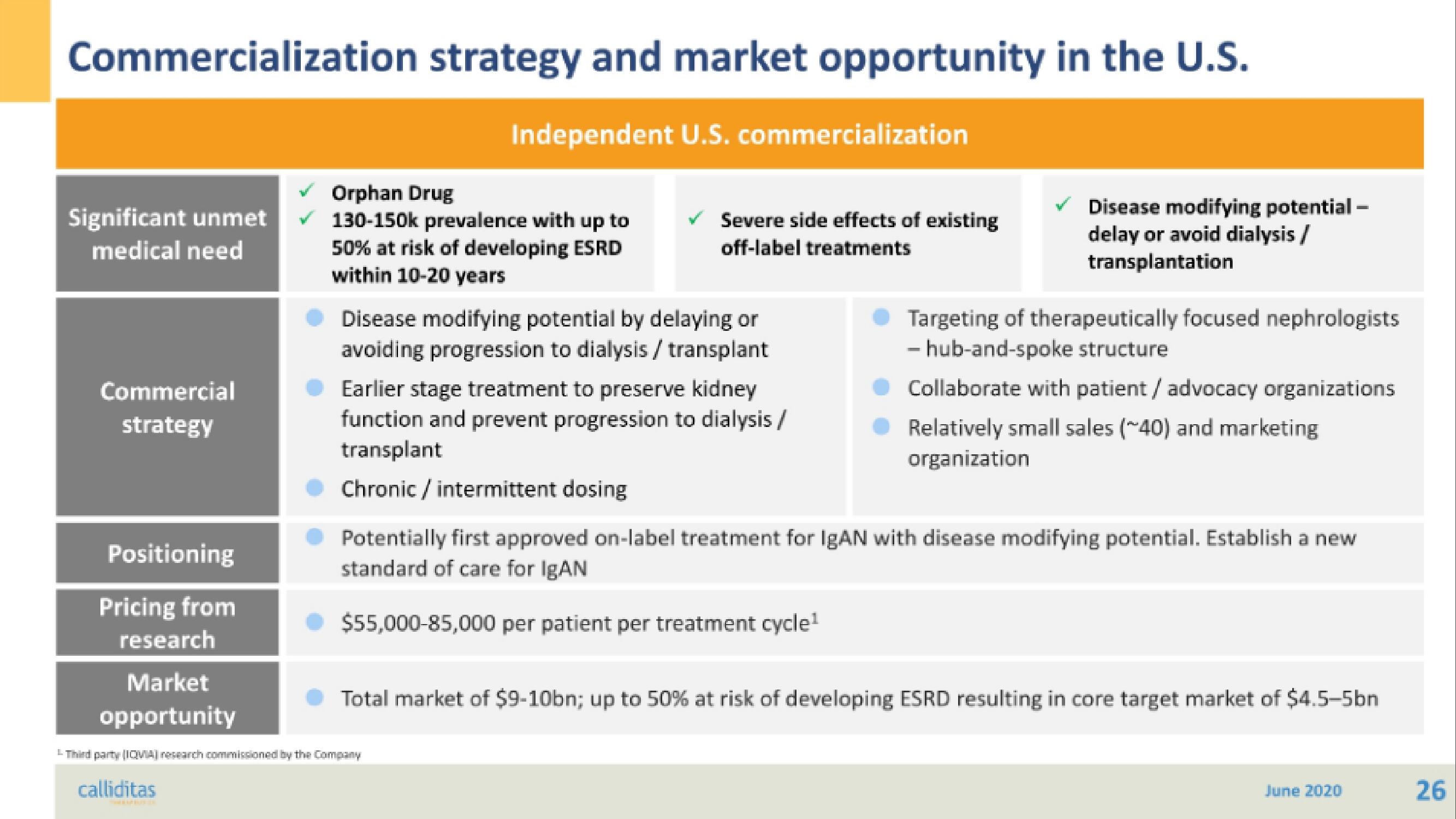
Significant unmet
medical need
Commercial
strategy
Positioning
Pricing from
research
Market
opportunity
calliditas
Independent U.S. commercialization
Orphan Drug
130-150k prevalence with up to
50% at risk of developing ESRD
within 10-20 years
www.
Third party IQVIA) research commissioned by the Company
Severe side effects of existing
off-label treatments
Disease modifying potential by delaying or
avoiding progression to dialysis / transplant
Earlier stage treatment to preserve kidney
function and prevent progression to dialysis/
transplant
Chronic / intermittent dosing
✓ Disease modifying potential-
delay or avoid dialysis /
transplantation
Targeting of therapeutically focused nephrologists
- hub-and-spoke structure
Collaborate with patient / advocacy organizations
Relatively small sales (~40) and marketing
organization
Potentially first approved on-label treatment for IgAN with disease modifying potential. Establish a new
standard of care for IgAN
$55,000-85,000 per patient per treatment cycle¹
Total market of $9-10bn; up to 50% at risk of developing ESRD resulting in core target market of $4.5-5bn
June 2020
26View entire presentation