Matinas BioPharma Investor Presentation Deck
9
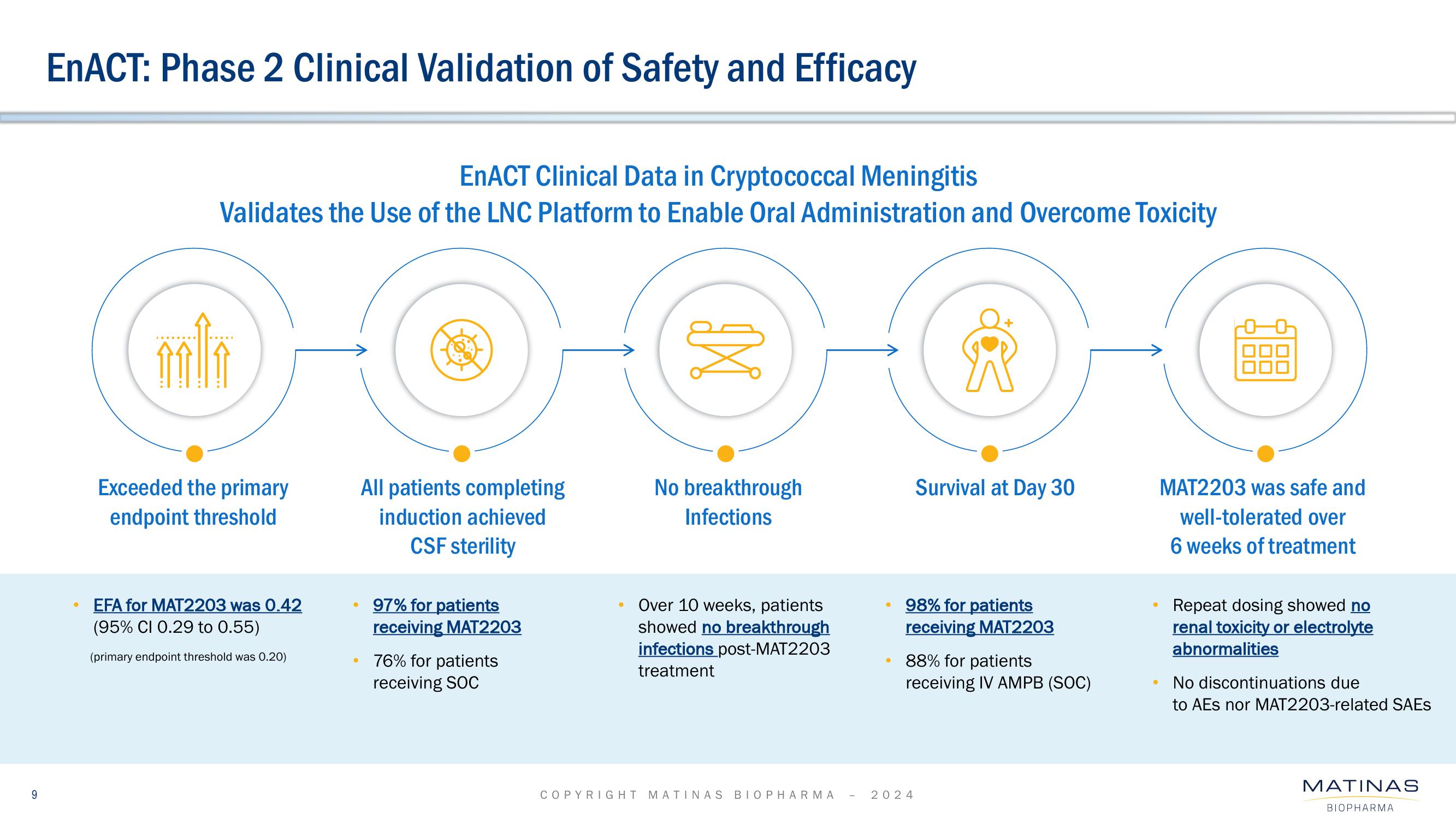
EnACT: Phase 2 Clinical Validation of Safety and Efficacy
EnACT Clinical Data in Cryptococcal Meningitis
Validates the Use of the LNC Platform to Enable Oral Administration and Overcome Toxicity
collo
Exceeded the primary
endpoint threshold
EFA for MAT2203 was 0.42
(95% CI 0.29 to 0.55)
(primary endpoint threshold was 0.20)
6
All patients completing
induction achieved
CSF sterility
97% for patients
receiving MAT2203
76% for patients
receiving SOC
Mi
No breakthrough
Infections
Over 10 weeks, patients
showed no breakthrough
infections post-MAT2203
treatment
COPYRIGHT MATINAS BIOPHARMA
●
+
Survival at Day 30
98% for patients
receiving MAT2203
2024
88% for patients
receiving IV AMPB (SOC)
Ⓡ
MAT2203 was safe and
well-tolerated over
6 weeks of treatment
Repeat dosing showed no
renal toxicity or electrolyte
abnormalities
No discontinuations due
to AEs nor MAT2203-related SAEs
MATINAS
BIOPHARMAView entire presentation