Valneva IPO Presentation Deck
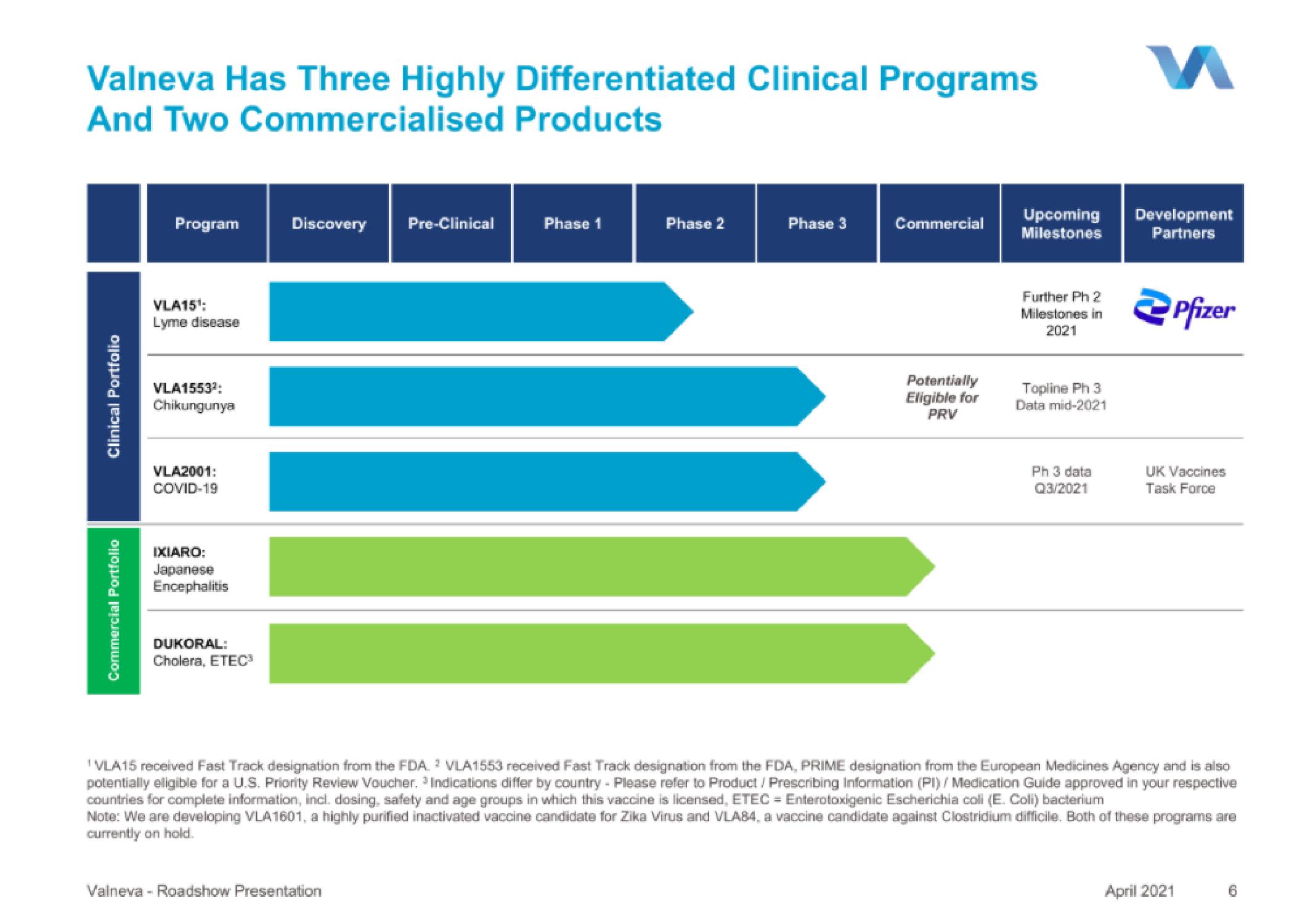
Valneva Has Three Highly Differentiated Clinical Programs
And Two Commercialised Products
Clinical Portfolio
Commercial Portfolio
Program
VLA15¹:
Lyme disease
VLA1553²:
Chikungunya
VLA2001:
COVID-19
IXIARO:
Japanese
Encephalitis
DUKORAL:
Cholera, ETEC³
Discovery Pre-Clinical
Phase 1
Valneva - Roadshow Presentation
Phase 2
Phase 3
Commercial
Potentially
Eligible for
PRV
Upcoming
Milestones
Further Ph 2
Milestones in
2021
Topline Ph 3
Data mid-2021
Ph 3 data
Q3/2021
M
Development
Partners
Pfizer
UK Vaccines
Task Force
VLA15 received Fast Track designation from the FDA. ² VLA1553 received Fast Track designation from the FDA, PRIME designation from the European Medicines Agency and is also
potentially eligible for a U.S. Priority Review Voucher. Indications differ by country - Please refer to Product / Prescribing Information (PI) / Medication Guide approved in your respective
countries for complete information, incl. dosing, safety and age groups in which this vaccine is licensed, ETEC = Enterotoxigenic Escherichia coli (E. Coli) bacterium
Note: We are developing VLA1601, a highly purified inactivated vaccine candidate for Zika Virus and VLA84, a vaccine candidate against Clostridium difficile. Both of these programs are
currently on hold.
April 2021View entire presentation