Ocuphire Pharma Results
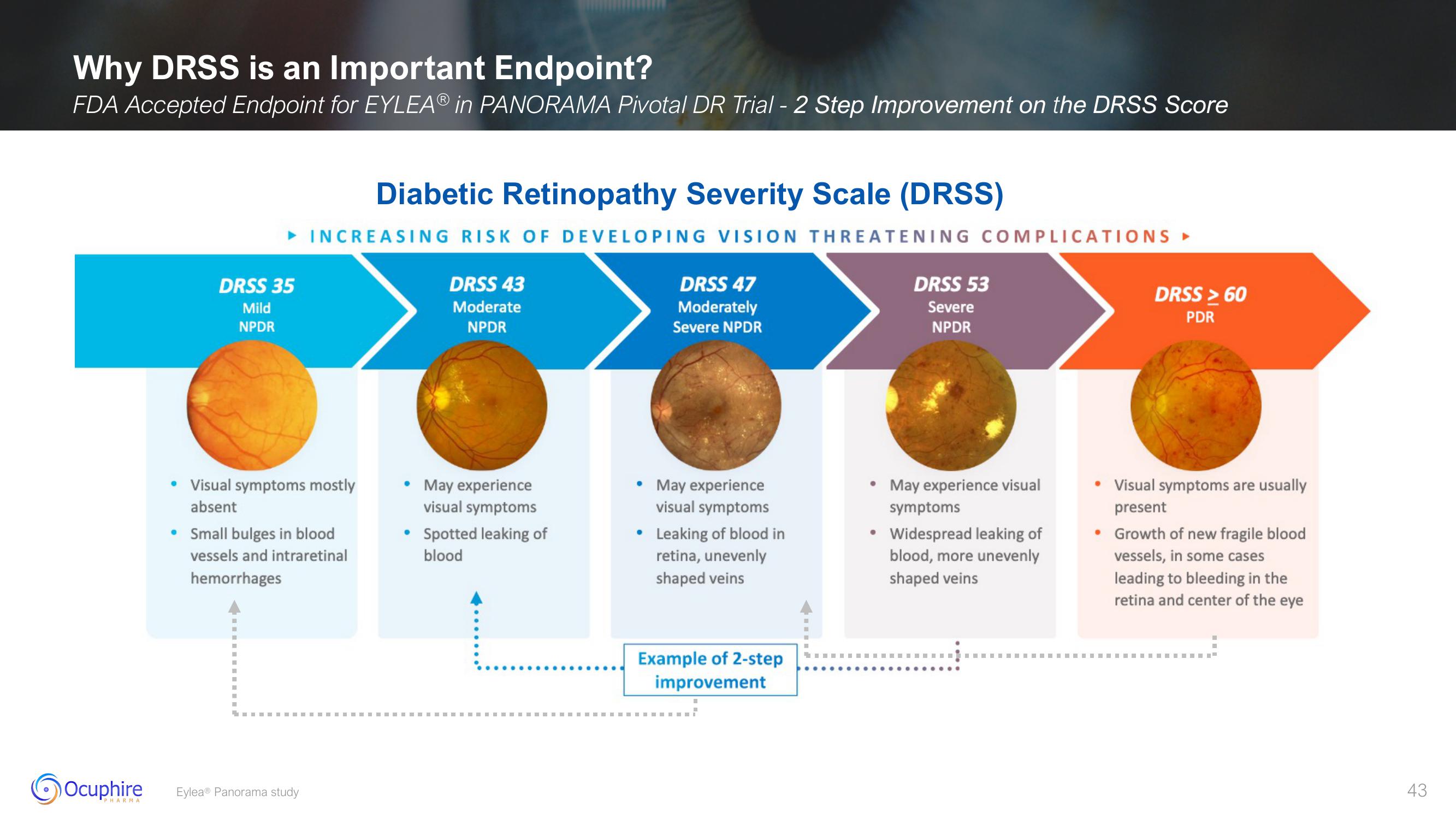
Why DRSS is an Important Endpoint?
FDA Accepted Endpoint for EYLEAⓇ in PANORAMA Pivotal DR Trial - 2 Step Improvement on the DRSS Score
Diabetic Retinopathy Severity Scale (DRSS)
► INCREASING RISK OF DEVELOPING VISION THREATENING COMPLICATIONS ►
DRSS 35
Mild
NPDR
• Visual symptoms mostly
absent
• Small bulges in blood
vessels and intraretinal
hemorrhages
■
Ocuphire Eylea® Panorama study
PHARMA
DRSS 43
Moderate
NPDR
• May experience
visual symptoms
Spotted leaking of
blood
●
¯¯¯¯¯¯¯¯¯¯¯
DRSS 47
Moderately
Severe NPDR
• May experience
visual symptoms
• Leaking of blood in
retina, unevenly
shaped veins
Example of 2-step
improvement
DRSS 53
Severe
NPDR
• May experience visual
symptoms
Widespread leaking of
blood, more unevenly
shaped veins
DRSS > 60
PDR
• Visual symptoms are usually
present
• Growth of new fragile blood
vessels, in some cases
leading to bleeding in the
retina and center of the eye
■
43View entire presentation