BioAtla IPO Presentation Deck
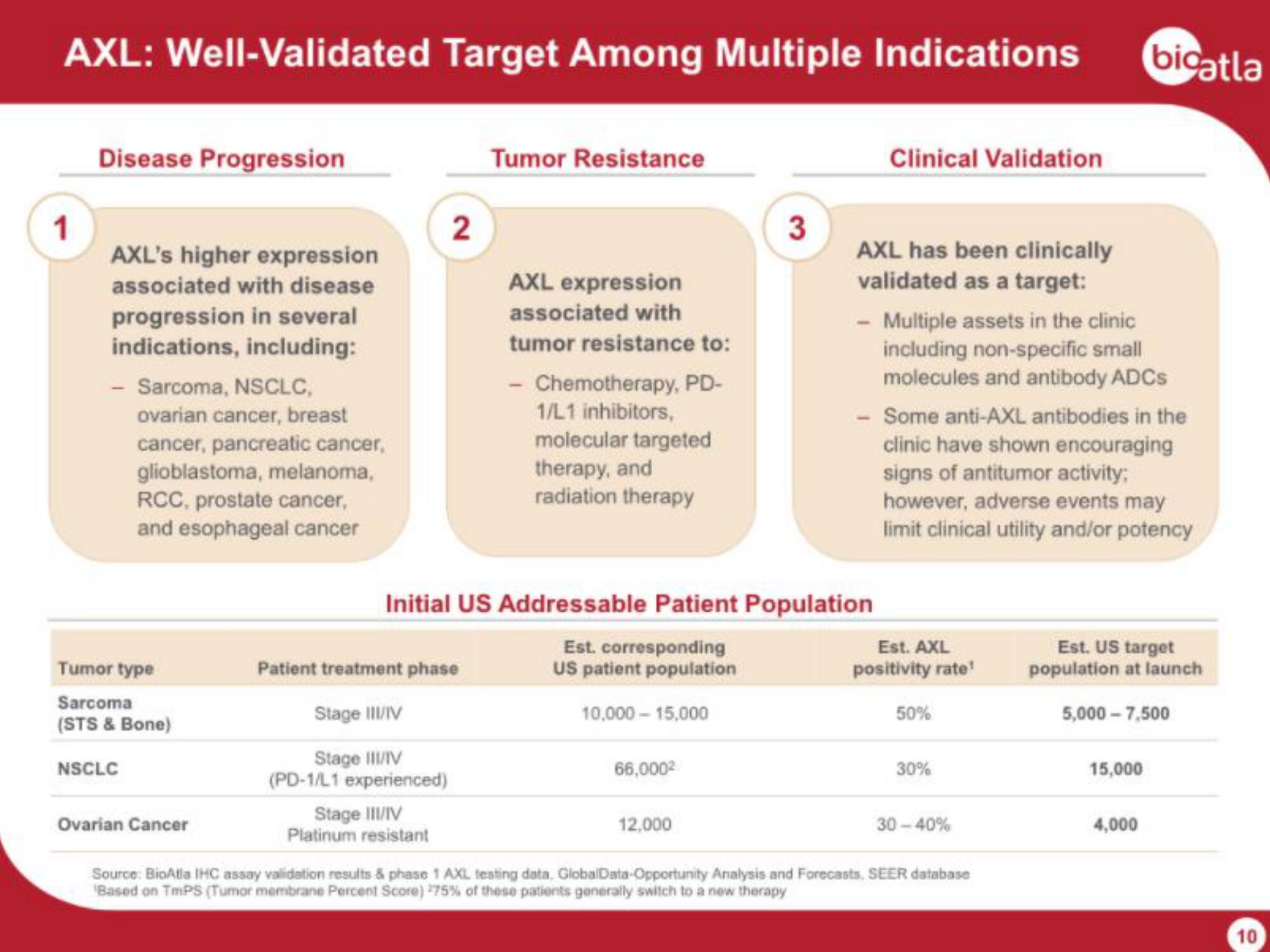
AXL: Well-Validated Target Among Multiple Indications
1
Disease Progression
AXL's higher expression
associated with disease
progression in several
indications, including:
- Sarcoma, NSCLC,
ovarian cancer, breast
cancer, pancreatic cancer,
glioblastoma, melanoma,
RCC, prostate cancer,
and esophageal cancer
Tumor type
Sarcoma
(STS & Bone)
NSCLC
Ovarian Cancer
2
Patient treatment phase
Stage III/IV
Stage III/IV
(PD-1/L1 experienced)
Stage III/IV
Platinum resistant
Tumor Resistance
AXL expression
associated with
tumor resistance to:
- Chemotherapy, PD-
1/L1 inhibitors,
molecular targeted
therapy, and
radiation therapy
10,000-15,000
Initial US Addressable Patient Population
Est. corresponding
US patient population
66,000²
3
12,000
Clinical Validation
AXL has been clinically
validated as a target:
- Multiple assets in the clinic
including non-specific small
molecules and antibody ADCs
- Some anti-AXL antibodies in the
clinic have shown encouraging
signs of antitumor activity:
however, adverse events may
limit clinical utility and/or potency
Est. AXL
positivity rate¹
50%
30%
30-40%
Source: BioAtla IHC assay validation results & phase 1 AXL testing data, GlobalData-Opportunity Analysis and Forecasts, SEER database
Based on TmPS (Tumor membrane Percent Score) 275% of these patients generally switch to a new therapy
bigatla
Est. US target
population at launch
5,000-7,500
15,000
4,000
10View entire presentation