Equillium Results Presentation Deck
Interim Safety Data Summary - Type B
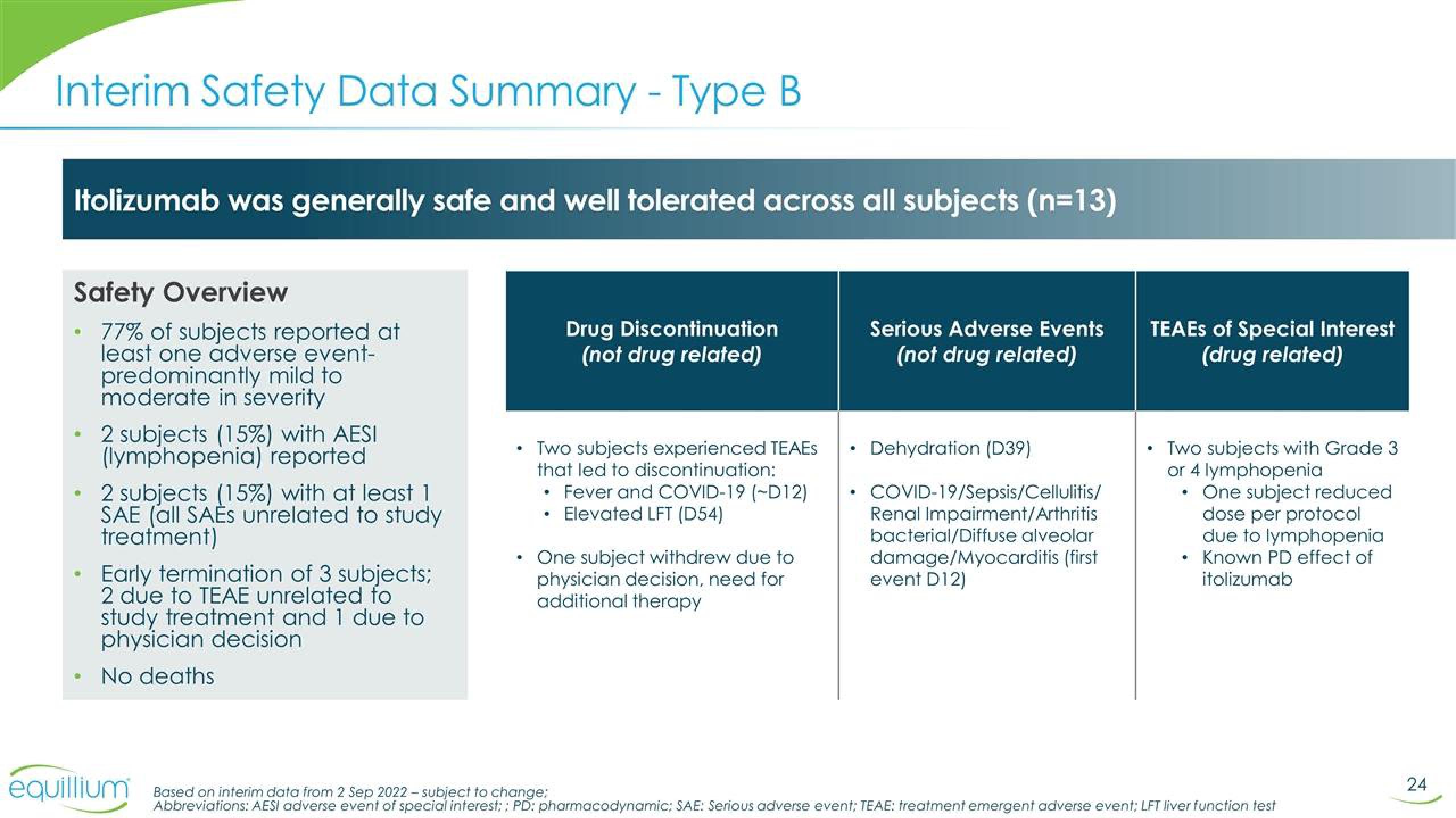
Itolizumab was generally safe and well tolerated across all subjects (n=13)
Safety Overview
77% of subjects reported at
least one adverse event-
predominantly mild to
moderate in severity
2 subjects (15%) with AESI
(lymphopenia) reported
2 subjects (15%) with at least 1
SAE (all SAEs unrelated to study
treatment)
Early termination of 3 subjects;
2 due to TEAE unrelated fo
study treatment and 1 due to
physician decision
No deaths
equillium
☐
Drug Discontinuation
(not drug related)
Two subjects experienced TEAES
that led to discontinuation:
Fever and COVID-19 (-D12)
Elevated LFT (D54)
•
One subject withdrew due to
physician decision, need for
additional therapy
Serious Adverse Events
(not drug related)
Dehydration (D39)
COVID-19/Sepsis/Cellulitis/
Renal Impairment/Arthritis
bacterial/Diffuse alveolar
damage/Myocarditis (first
event D12)
TEAES of Special Interest
(drug related)
Two subjects with Grade 3
or 4 lymphopenia
✔
*
One subject reduced
dose per protocol
due to lymphopenia
Known PD effect of
itolizumab
Based on interim data from 2 Sep 2022-subject to change;
Abbreviations: AESI adverse event of special interest:: PD: pharmacodynamic: SAE: Serious adverse event; TEAE: treatment emergent adverse event: LFT liver function test
24View entire presentation