Calliditas Therapeutics IPO Presentation Deck
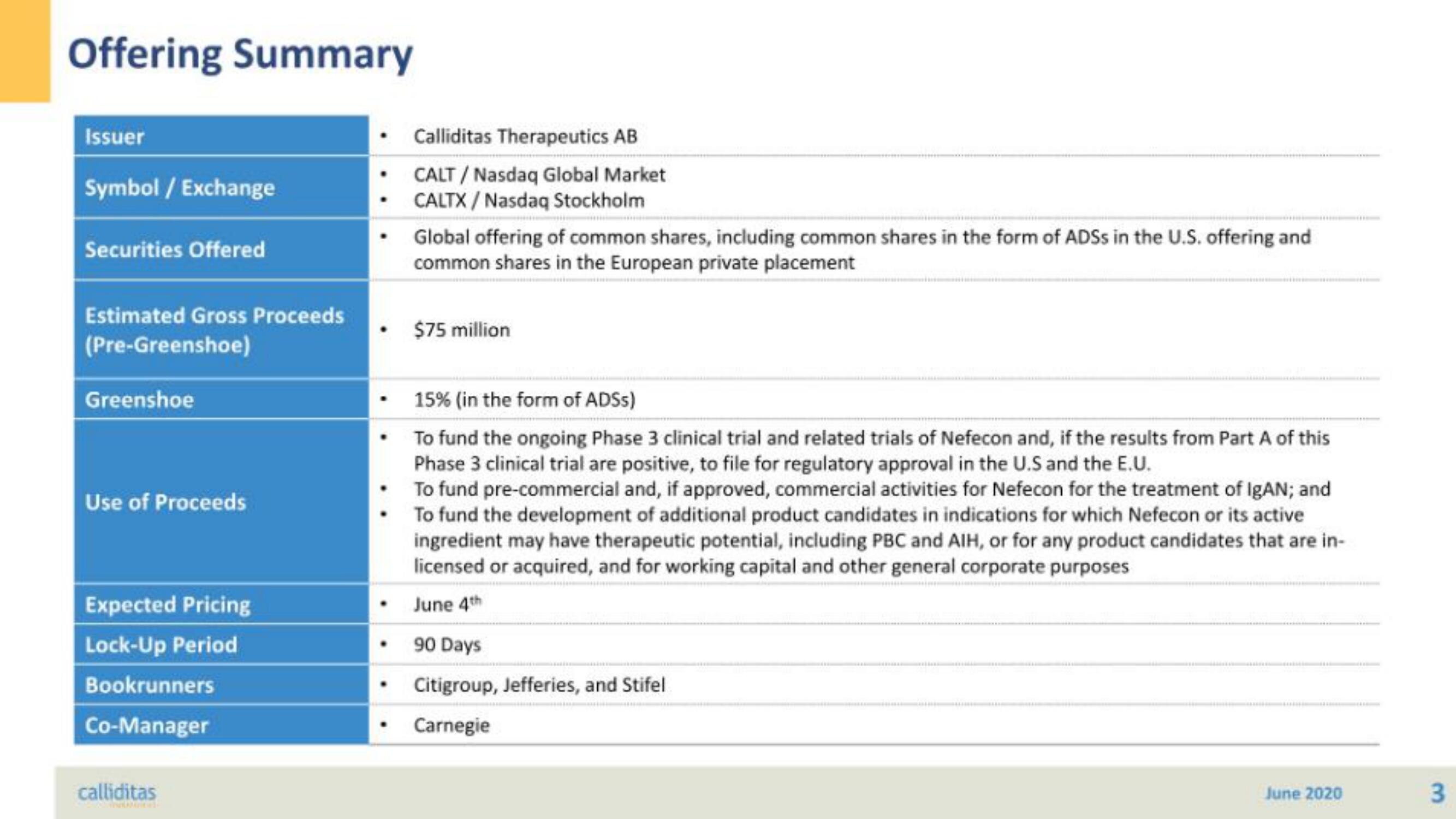
Offering Summary
Issuer
Symbol / Exchange
Securities Offered
Estimated Gross Proceeds
(Pre-Greenshoe)
Greenshoe
Use of Proceeds
Expected Pricing
Lock-Up Period
Bookrunners
Co-Manager
calliditas
Calliditas Therapeutics AB
CALT/Nasdaq Global Market
CALTX/Nasdaq Stockholm
Global offering of common shares, including common shares in the form of ADSS in the U.S. offering and
common shares in the European private placement
$75 million
15% (in the form of ADSS)
To fund the ongoing Phase 3 clinical trial and related trials of Nefecon and, if the results from Part A of this
Phase 3 clinical trial are positive, to file for regulatory approval in the U.S and the E.U.
To fund pre-commercial and, if approved, commercial activities for Nefecon for the treatment of IgAN; and
To fund the development of additional product candidates in indications for which Nefecon or its active
ingredient may have therapeutic potential, including PBC and AIH, or for any product candidates that are in-
licensed or acquired, and for working capital and other general corporate purposes
June 4th
90 Days
Citigroup, Jefferies, and Stifel
Carnegie
June 2020
3View entire presentation