Valneva IPO Presentation Deck
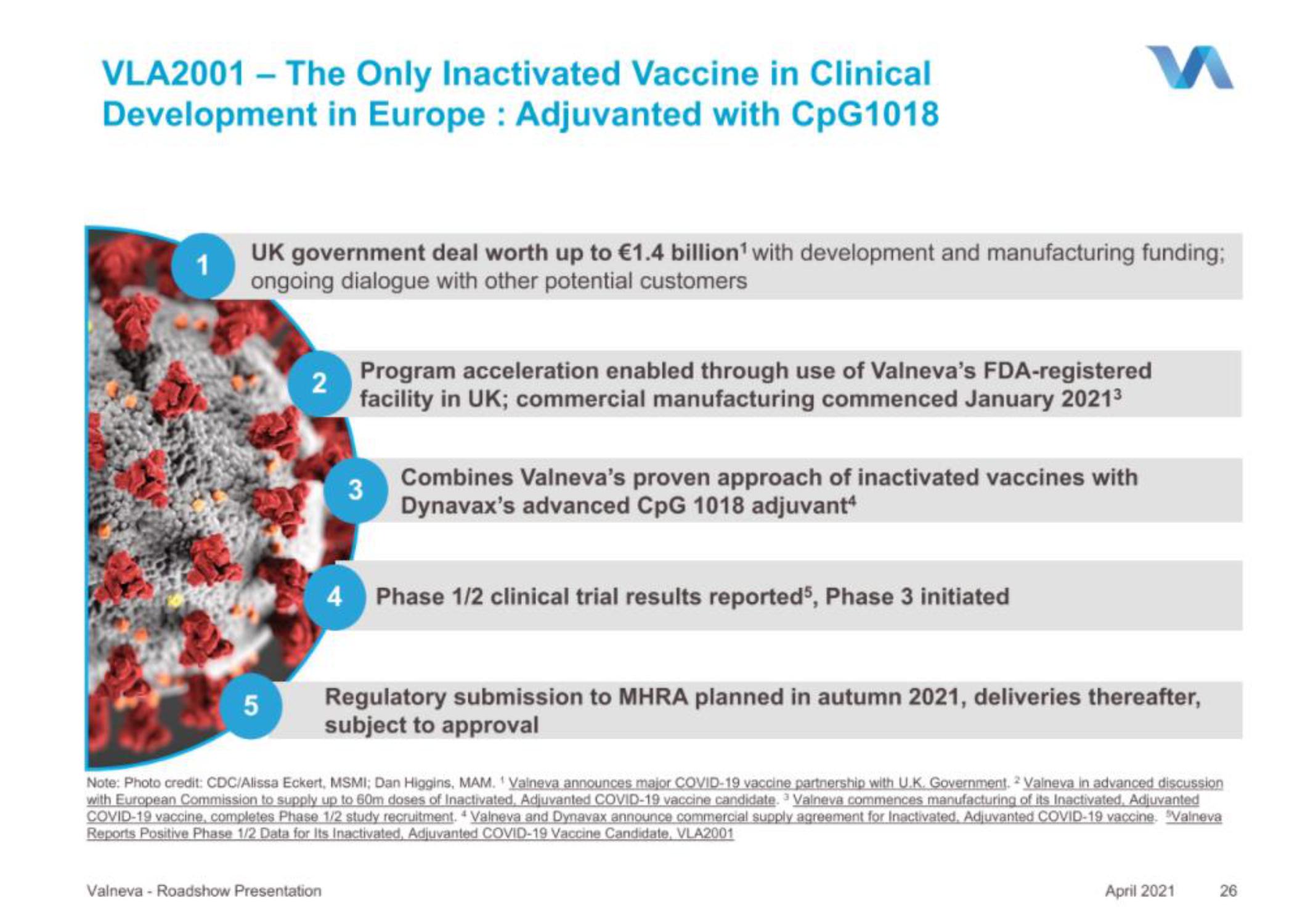
VLA2001 - The Only Inactivated Vaccine in Clinical
Development in Europe: Adjuvanted with CpG1018
1
UK government deal worth up to €1.4 billion¹ with development and manufacturing funding;
ongoing dialogue with other potential customers
5
2
Program acceleration enabled through use of Valneva's FDA-registered
facility in UK; commercial manufacturing commenced January 2021³
Valneva - Roadshow Presentation
3
Combines Valneva's proven approach of inactivated vaccines with
Dynavax's advanced CpG 1018 adjuvant
Phase 1/2 clinical trial results reported5, Phase 3 initiated
Regulatory submission to MHRA planned in autumn 2021, deliveries thereafter,
subject to approval
Note: Photo credit: CDC/Alissa Eckert, MSMI; Dan Higgins, MAM. Valneva announces major COVID-19 vaccine partnership with U.K. Government. Vaineva in advanced discussion
with European Commission to supply up to 60m doses of Inactivated, Adjuvanted COVID-19 vaccine candidate. ³ Valneva commences manufacturing of its Inactivated, Adjuvanted
COVID-19 vaccine, completes Phase 1/2 study recruitment. Valneva and Dynavax announce commercial supply agreement for Inactivated, Adjuvanted COVID-19 vaccine. "Vaineva
Reports Positive Phase 1/2 Data for Its Inactivated, Adjuvanted COVID-19 Vaccine Candidate, VLA2001
April 2021
26View entire presentation