Kymera Investor Presentation Deck
●
●
●
●
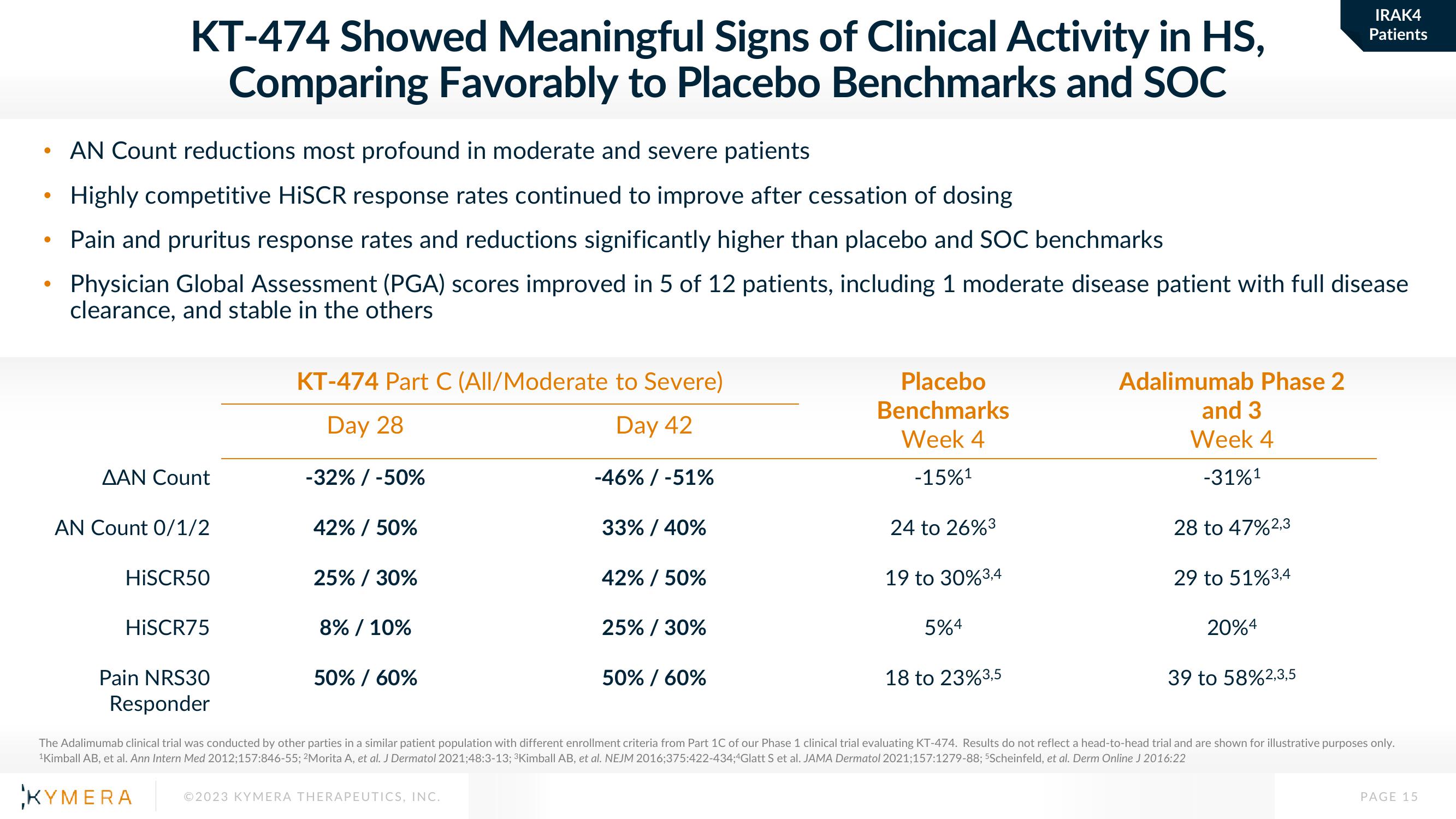
KT-474 Showed Meaningful Signs of Clinical Activity in HS,
Comparing Favorably to Placebo Benchmarks and SOC
AN Count reductions most profound in moderate and severe patients
Highly competitive HISCR response rates continued to improve after cessation of dosing
Pain and pruritus response rates and reductions significantly higher than placebo and SOC benchmarks
Physician Global Assessment (PGA) scores improved in 5 of 12 patients, including 1 moderate disease patient with full disease
clearance, and stable in the others
ΔΑΝ Count
AN Count 0/1/2
HISCR50
HISCR75
Pain NRS30
Responder
KT-474 Part C (All/Moderate to Severe)
Day 28
Day 42
-32% / -50%
42% / 50%
25% / 30%
8% / 10%
50% / 60%
-46% / -51%
33% / 40%
42% / 50%
25% / 30%
50% / 60%
Placebo
Benchmarks
Week 4
-15%¹
24 to 26%3
19 to 30% 3,4
5%4
18 to 23% 3,5
Adalimumab Phase 2
and 3
Week 4
-31%¹
28 to 47%2,3
29 to 51% 3,4
20%4
IRAK4
Patients
39 to 58%2,3,5
The Adalimumab clinical trial was conducted by other parties in a similar patient population with different enrollment criteria from Part 1C of our Phase 1 clinical trial evaluating KT-474. Results do not reflect a head-to-head trial and are shown for illustrative purposes only.
¹Kimball AB, et al. Ann Intern Med 2012;157:846-55; 2Morita A, et al. J Dermatol 2021;48:3-13; ³Kimball AB, et al. NEJM 2016;375:422-434;4Glatt S et al. JAMA Dermatol 2021;157:1279-88; 5Scheinfeld, et al. Derm Online J 2016:22
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
PAGE 15View entire presentation