Centessa IPO Presentation Deck
Lixivaptan: Phase 2 Results Support Potential in ADPKD
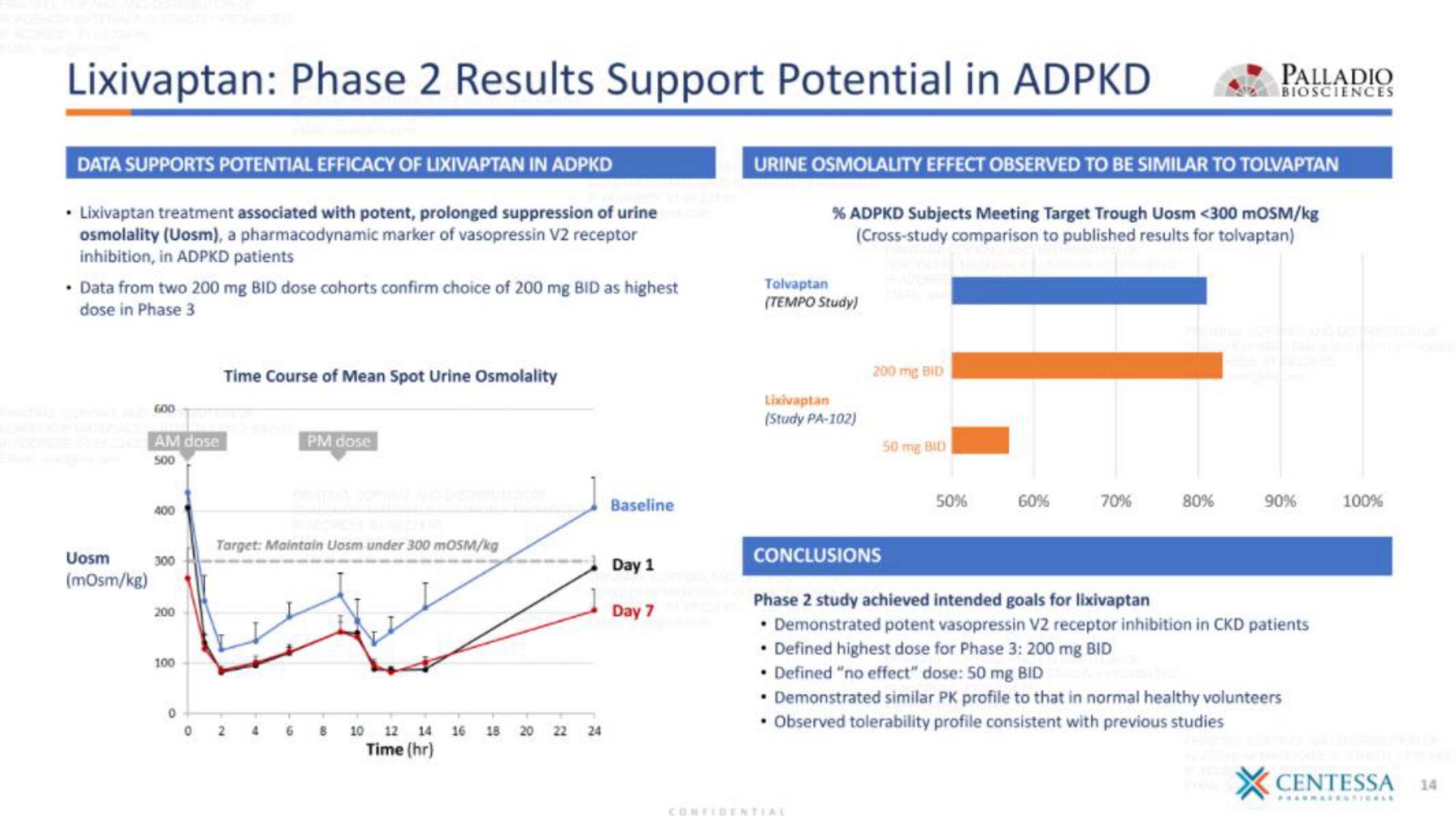
DATA SUPPORTS POTENTIAL EFFICACY OF LIXIVAPTAN IN ADPKD
• Lixivaptan treatment associated with potent, prolonged suppression of urine
osmolality (Uosm), a pharmacodynamic marker of vasopressin V2 receptor
inhibition, in ADPKD patients
• Data from two 200 mg BID dose cohorts confirm choice of 200 mg BID as highest
dose in Phase 3
Uosm
(mOsm/kg)
600
AM dose
500
400
300
200
100
0
0
Time Course of Mean Spot Urine Osmolality
PM dose
Target: Maintain Uasm under 300 mOSM/kg
10 12 14 16 18
Time (hr)
20 22 24
Baseline
Day 1
Day 7
URINE OSMOLALITY EFFECT OBSERVED TO BE SIMILAR TO TOLVAPTAN
% ADPKD Subjects Meeting Target Trough Uosm <300 mOSM/kg
(cross-study comparison to published results for tolvaptan)
Tolvaptan
(TEMPO Study)
Lixivaptan
(Study PA-102)
200 mg BID
50 mg BID
50%
60%
70%
PALLADIO
BIOSCIENCES
80%
90%
CONCLUSIONS
Phase 2 study achieved intended goals for lixivaptan
. Demonstrated potent vasopressin V2 receptor inhibition in CKD patients
• Defined highest dose for Phase 3: 200 mg BID
• Defined "no effect" dose: 50 mg BID
• Demonstrated similar PK profile to that in normal healthy volunteers
• Observed tolerability profile consistent with previous studies
100%
CENTESSA 14View entire presentation