AstraZeneca Results Presentation Deck
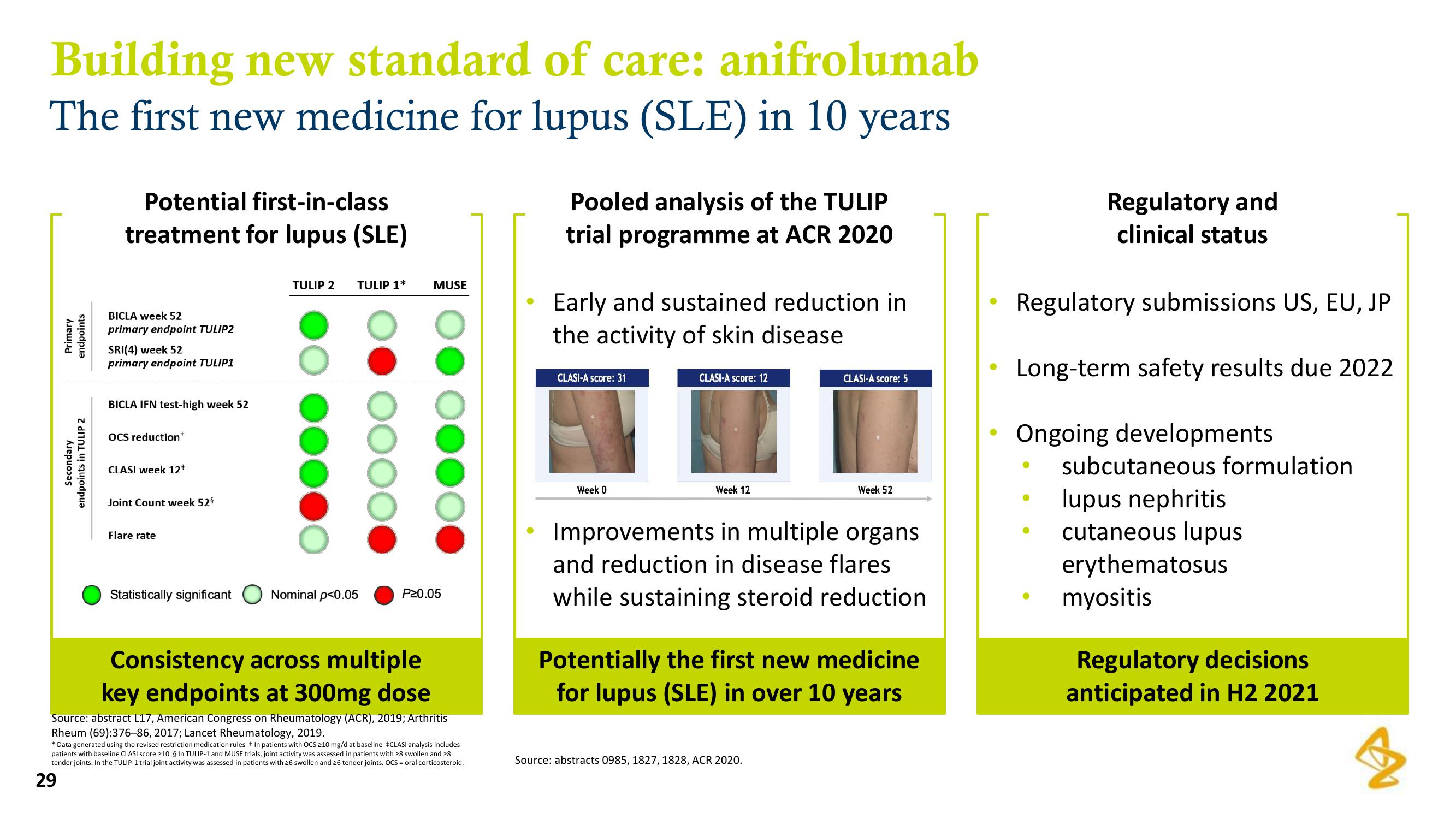
Building new standard of care: anifrolumab
The first new medicine for lupus (SLE) in 10 years
Primary
endpoints
Secondary
endpoints in TULIP 2
Potential first-in-class
treatment for lupus (SLE)
BICLA week 52
primary endpoint TULIP2
SRI(4) week 52
primary endpoint TULIP1
BICLA IFN test-high week 52
OCS reduction+
CLASI week 12*
Joint Count week 52⁹
Flare rate
Statistically significant
TULIP 2 TULIP 1*
Nominal p<0.05
MUSE
P20.05
Consistency across multiple
key endpoints at 300mg dose
Source: abstract L17, American Congress on Rheumatology (ACR), 2019; Arthritis
Rheum (69):376-86, 2017; Lancet Rheumatology, 2019.
* Data generated using the revised restriction medication rules + In patients with OCS 210 mg/d at baseline #CLASI analysis includes
patients with baseline CLASI score 210 § In TULIP-1 and MUSE trials, joint activity was assessed in patients with 28 swollen and 28
tender joints. In the TULIP-1 trial joint activity was assessed in patients with 26 swollen and 26 tender joints. OCS = oral corticosteroid.
29
Pooled analysis of the TULIP
trial programme at ACR 2020
Early and sustained reduction in
the activity of skin disease
CLASI-A score: 31
Week 0
CLASI-A score: 12
Week 12
CLASI-A score: 5
Week 52
Improvements in multiple organs
and reduction in disease flares
while sustaining steroid reduction
Source: abstracts 0985, 1827, 1828, ACR 2020.
Potentially the first new medicine
for lupus (SLE) in over 10 years
Regulatory and
clinical status
Regulatory submissions US, EU, JP
Long-term safety results due 2022
Ongoing developments
subcutaneous formulation
lupus nephritis
cutaneous lupus
erythematosus
myositis
Regulatory decisions
anticipated in H2 2021
3View entire presentation