Imara M&A
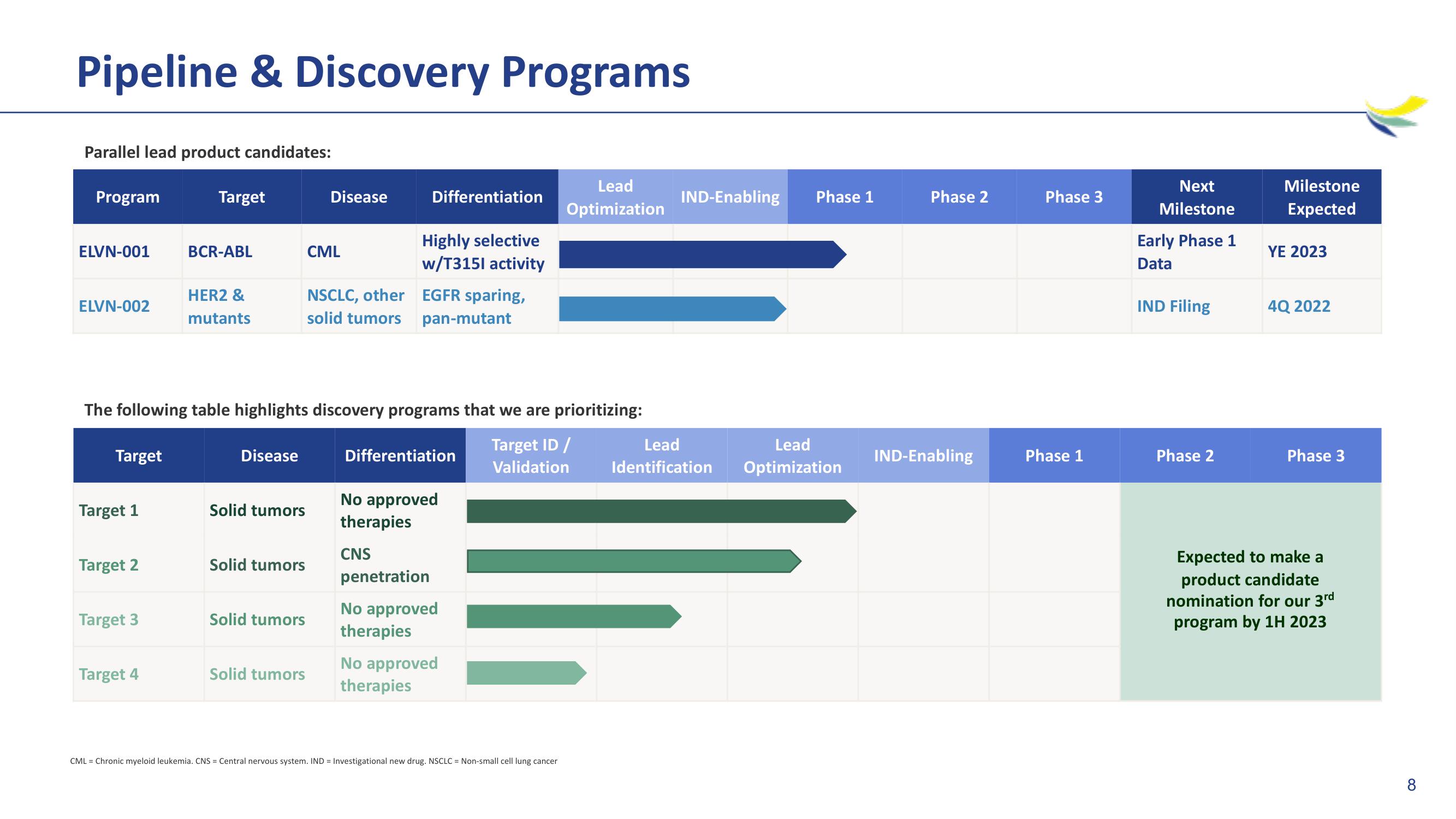
Pipeline & Discovery Programs
Parallel lead product candidates:
Program
ELVN-001
ELVN-002
Target
Target 1
Target 2
Target 3
Target
Target 4
BCR-ABL
HER2 &
mutants
The following table highlights discovery programs that we are prioritizing:
Target ID /
Validation
Disease
Solid tumors
Solid tumors
Solid tumors
Disease
Solid tumors
CML
NSCLC, other
solid tumors
Differentiation
Highly selective
w/T3151 activity
EGFR sparing,
pan-mutant
Differentiation
No approved
therapies
CNS
penetration
No approved
therapies
No approved
therapies
Lead
Optimization
CML = Chronic myeloid leukemia. CNS = Central nervous system. IND = Investigational new drug. NSCLC = Non-small cell lung cancer
IND-Enabling
Lead
Identification
Phase 1
Lead
Optimization
Phase 2
IND-Enabling
Phase 3
Phase 1
Next
Milestone
Early Phase 1
Data
IND Filing
Phase 2
Milestone
Expected
YE 2023
4Q 2022
Phase 3
Expected to make a
product candidate
nomination for our 3rd
program by 1H 2023
8View entire presentation