BioAtla Investor Conference Presentation Deck
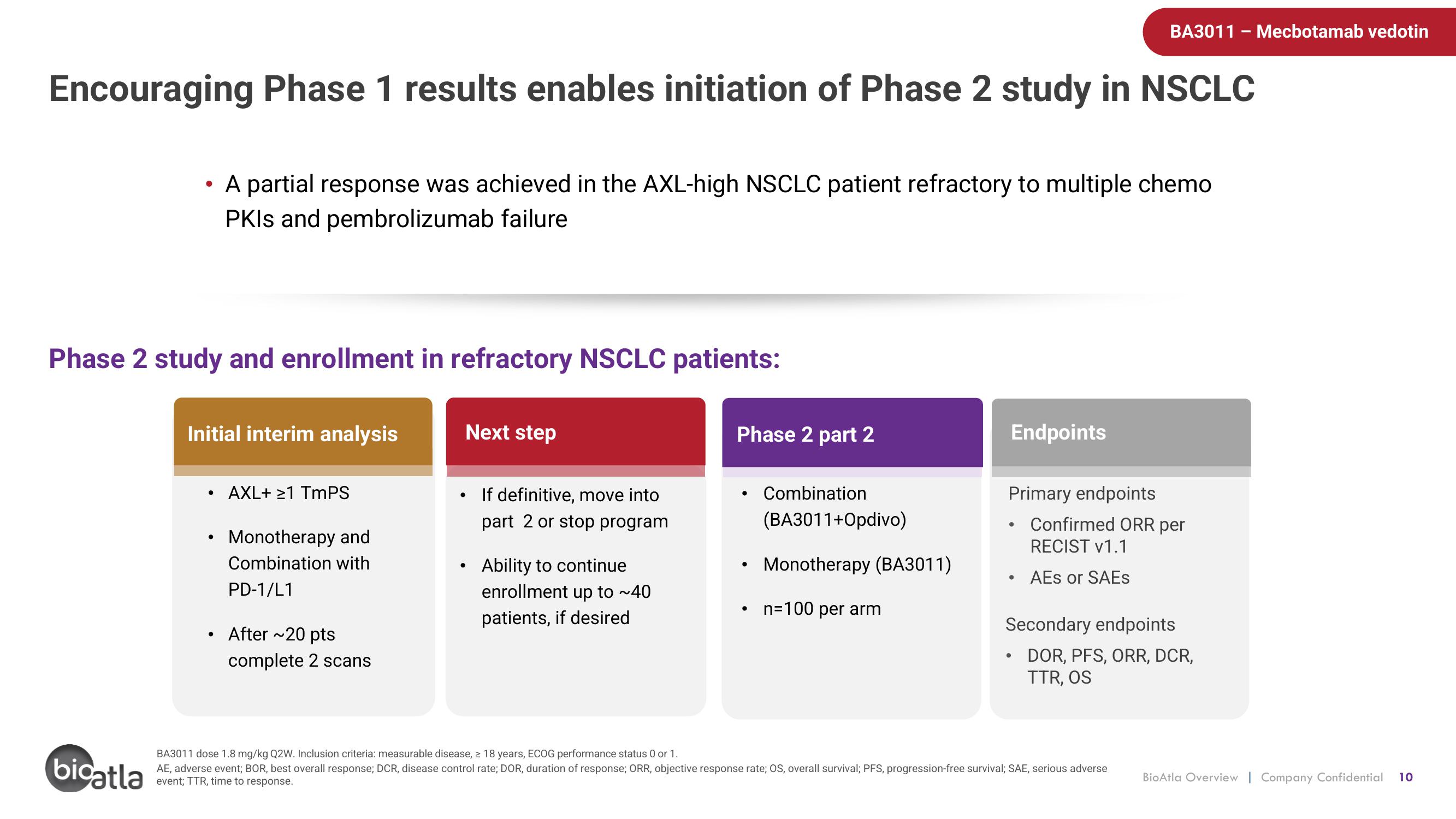
Encouraging Phase 1 results enables initiation of Phase 2 study in NSCLC
Phase 2 study and enrollment in refractory NSCLC patients:
bicatla
●
A partial response was achieved in the AXL-high NSCLC patient refractory to multiple chemo
PKIs and pembrolizumab failure
Initial interim analysis
●
●
●
AXL+ ≥1 TmPS
Monotherapy and
Combination with
PD-1/L1
After ~20 pts
complete 2 scans
Next step
●
●
If definitive, move into
part 2 or stop program
Ability to continue
enrollment up to ~40
patients, if desired
Phase 2 part 2
●
●
●
Combination
(BA3011+Opdivo)
Monotherapy (BA3011)
n=100 per arm
Endpoints
Primary endpoints
●
●
BA3011 - Mecbotamab vedotin
●
Confirmed ORR per
RECIST v1.1
AES or SAEs
Secondary endpoints
DOR, PFS, ORR, DCR,
TTR, OS
BA3011 dose 1.8 mg/kg Q2W. Inclusion criteria: measurable disease, ≥ 18 years, ECOG performance status 0 or 1.
AE, adverse event; BOR, best overall response; DCR, disease control rate; DOR, duration of response; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; SAE, serious adverse
event; TTR, time to response.
BioAtla Overview | Company Confidential
10View entire presentation